The Wnt signaling pathway, an evolutionarily conserved signal transduction pathway, is widely present in invertebrates and vertebrates. The Wnt signaling pathway plays a crucial role in early embryonic development, organogenesis, tissue repair, and many other physiological processes. The mutation of key proteins involved in this pathway can lead to abnormal activation of signals, and potentially induces the occurrence of cancer. In 1982, R. Nusse and H.E. Varmus identified the first Wnt gene from a mouse mammary tumor and named it Int1 (integration 1). Continued research found that the mouse Int and Drosophila Wingless (Wg) genes are homeotic, and thus combined their names to Wnt. H.E. Varmus himself also won the 1989 Nobel Prize in Physiology or Medicine for his great contribution in oncology.
Wnt comprises a diverse family of secreted glycoproteins that act via paracrine (cell-cell) or autocrine (same-cell) signaling. By binding to specific receptors present on receiving cells, secreted Wnts initiate phosphorylation and dephosphorylation of downstream proteins, leading to the accumulation of β-catenin. β-catenin is a multi-functional protein that can bind to E-cadherin on the cellular membrane, creating a cell-cell adhesion complex. If not bound to E-cadherin, free β-catenin is able to enter the nucleus where it regulates gene expression. Therefore, the aberrant high expression of β-catenin may result in various diseases including cancer.
Source: ResearchGate
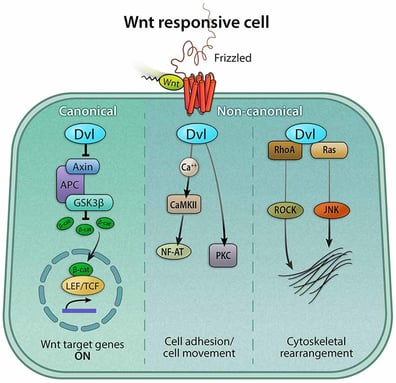
As a complicated regulation network, the Wnt signaling pathway has so far been characterized into three branches - one canonical and two non-canonical pathways:
1. The canonical Wnt pathway, activating gene transcription via β-catenin;
2. The Wnt/PCP pathway (planar cell polarity pathway), activating JNK (c-Jun N-terminal kinases) via G proteins to regulate cytoskeletal reorganization;
3. The Wnt/calcium pathway, regulating calcium release to control cell adhesion and targeted gene expression.
The canonical Wnt pathway (or Wnt/β-catenin pathway) is often referred to as the Wnt pathway for short.
1. Latest Progress
Recently, many scientists have devoted a great amount of work to unveiling this pathway.
Researchers from the University of Leeds found that the vitamin D receptor (VDR) can inhibit melanoma progression and promote anti-tumor immunity.[1] Published in Cancer Research in November 2019, the article confirmed that adding VDR on mouse melanoma cells deactivated the Wnt/β-catenin pathway, decelerated melanoma development, and inhibited cancer cells from migrating to the lung in mice.
Another research article published in Cancer Research in August 2019 proved that RUNX1 promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway and EMT in colorectal cancer (CRC).[2] RUNX1 plays the roles of an oncogene and an anti-oncogene in epithelial tumors, and abnormally elevated RUNX1 has been suggested to contribute to the carcinogenesis of CRC. RUNX1 expression is upregulated in CRC tissues, and upregulated RUNX1 promotes cell metastasis and EMT of CRC. According to said research, RUNX1 can activate Wnt/β-catenin signaling in CRC cells by directly interacting with β-catenin and targeting the promoter and enhancer regions of KIT to promote KIT transcription.
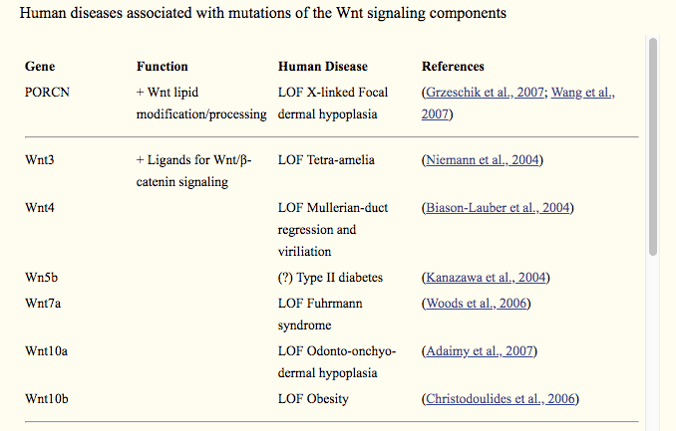
Mutations of the Wnt signaling components were also found associated with more than 20 human diseases, including type II diabetes, obesity, Wilms' tumor, cancer, and multiple types of birth defects. Table below shows the human diseases and their associated genes, from an article published in Developmental Cell in July 2009.[3]
*LOF: loss-of-function; GOF: gain-of-function. Source: PubMed
2. Wnt/β-Catenin Pathway Mechanisms
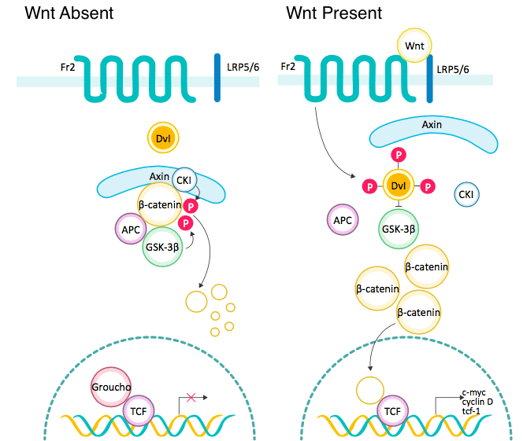
Source: ABclonal
In the absence of a Wnt signal, most cytoplasmic β-catenin binds to E-cadherin on the cellular membrane to maintain cell-cell adhesion. A small amount of β-catenin is phosphorylated by CK1 and targeted by the APC/Axin/GSK-3β complex, leading to its ubiquitination and proteasomal degradation. In the case that the Wnt/β-catenin pathway is activated, the Wnt ligand binds to Fzd receptors to form a complex that leads to activation of Dvl. Activated Dvl triggers displacement of GSK-3β from the APC/Axin/GSK-3β complex - a β-catenin destruction complex, and consequently stabilizes free cytoplasmic β-catenin. Accumulated β-catenin is translocated to the nucleus, where it binds to TCF/LEF transcription factors, regulating expression of target genes. For example, it can activate oncogenes including CyclinD1 and c-Myc, leading to cell proliferation, classification, and maturation.
In short, we conclude that the Wnt/β-catenin signaling pathway can be divided into the following steps:
Wnt → Fzd → Dvl → β-catenin destruction complex dissolved → β-catenin enters nucleus → TCF/LEF → downstream gene transcription
3. Main Components in Wnt/β-Catenin Pathway
1. Frizzled (Fzd or Frz)
Frizzled receptors are seven transmembrane G protein-coupled receptors for the Wnt family. Each Frizzled protein contains a N-terminal extracellular cysteine-rich domain (CRD), through which Wnt ligands bind to Frizzled receptors.
2. Dishevelled (Dsh or Dvl)
Acting downstream of a Frizzled receptor, Dishevelled inhibits the formation of a β-catenin destruction complex made of APC, Axin and GSK3β, and thus stabilizes cytosolic β-catenin. Accumulated β-catenin enters the nucleus, where it binds to the TCF/LEF family of transcription factors to activate target genes.
3. GSK3β
Glycogen synthase kinase 3 beta (GSK3β) is a serine-threonine protein kinase. With the absence of Wnt signal, GSK3β adds phosphate molecules onto serine-threonine residues of β-catenin. Phosphorylated β-catenin is recognized by the β-TrCP and subsequently degraded via the ubiquitin-dependent proteasome pathway.
4. CK1
Casein kinase 1 (CK1) phosphorylates the site Ser45 of β-catenin, “priming” the subsequent phosphorylation by GSK3β of residues Thr41, Ser37, and Ser33.
5. Axin
Axin is a scaffold protein that contains multiple domains for interaction with other proteins. It forms a β-catenin degradation complex along with APC, GSK3β and CK1. It also interacts with other Wnt signaling pathway components including Dishevelled and PP2A (protein phosphatase 2A).
6. TCF/LEF
The TCF/LEF family is a group of bi-directional transcription factors. When bound to Groucho, it inhibits gene transcription. Conversely, it can promote transcription of target genes if bound to β-catenin.
Wnt5a Rabbit mAb (A19133)
|
|
References