The term apoptosis was first used in 1972 to describe a morphologically distinct form of cell death. Since those early experiments and observations, apoptosis has become one of the focal points for biological research, with myriad laboratories and research groups continuing to work to further elucidate the components and pathways that drive this programmed cell death. As a fundamental biological pathway, apoptosis has benefits and adverse effects for the host organism. For example, many therapeutic strategies involve the activation of apoptosis to kill cancer cells, while other treatments seek to prevent apoptosis to preserve precious cells in key tissues.
The term apoptosis was first used in 1972 to describe a morphologically distinct form of cell death. Since those early experiments and observations, apoptosis has become one of the focal points for biological research, with myriad laboratories and research groups continuing to work to further elucidate the components and pathways that drive this programmed cell death. As a fundamental biological pathway, apoptosis has benefits and adverse effects for the host organism. For example, many therapeutic strategies involve the activation of apoptosis to kill cancer cells, while other treatments seek to prevent apoptosis to preserve precious cells in key tissues.
Introduction to Apoptosis
Apoptosis is a process of controlled cellular death whereby the activation of specific death-signaling pathways leads to the elimination of cells. Morphologically, apoptotic cells display a characteristic cytoplasmic cell shrinkage, budding of the plasma membrane, the presentation of phosphatidylserine (PS) on the extracellular surface, and chromatin condensation and DNA fragmentation. Since proinflammatory factors are usually not released, apoptosis is considered a non-inflammatory cell death.
The Extrinsic and Intrinsic Pathways of Apoptosis
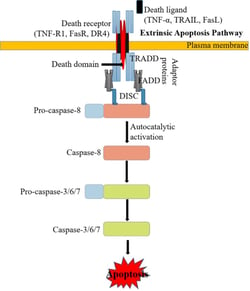 There are two main apoptotic pathways: the extrinsic (or death receptor) pathway and the intrinsic (or mitochondrial) pathway. Both pathways converge to activate the effector caspases, resulting in morphological and biochemical cellular alterations. The extrinsic (death receptor-dependent) apoptotic pathway (Figure 1) is initiated by the interaction of ligands with death receptors, belonging to the superfamily of tumor necrosis factor receptor (TNFR). The best-characterized ligands and corresponding death receptors include FasL/Fas, TNF-α/TNFR1, Apo3L/DR3, Apo2L/DR4 and Apo2L/DR5. Upon ligand binding, the death receptor-associated adapter proteins (e.g. FADD and TRADD) recruit the initiator pro-caspase-8 and/or -10, resulting in the formation of the so-called death-inducing signaling complex (DISC), increasing the local concentration of pro-caspase and promoting the mutual auto-activation. Activated caspase-8/10 cleaves other pro-caspases (caspase-3/6/7) into active “executioner” caspases. These executioner caspases then cause degradation of a variety of cellular structures, such as the cytoskeleton and nucleus, leading to apoptotic cell death.
There are two main apoptotic pathways: the extrinsic (or death receptor) pathway and the intrinsic (or mitochondrial) pathway. Both pathways converge to activate the effector caspases, resulting in morphological and biochemical cellular alterations. The extrinsic (death receptor-dependent) apoptotic pathway (Figure 1) is initiated by the interaction of ligands with death receptors, belonging to the superfamily of tumor necrosis factor receptor (TNFR). The best-characterized ligands and corresponding death receptors include FasL/Fas, TNF-α/TNFR1, Apo3L/DR3, Apo2L/DR4 and Apo2L/DR5. Upon ligand binding, the death receptor-associated adapter proteins (e.g. FADD and TRADD) recruit the initiator pro-caspase-8 and/or -10, resulting in the formation of the so-called death-inducing signaling complex (DISC), increasing the local concentration of pro-caspase and promoting the mutual auto-activation. Activated caspase-8/10 cleaves other pro-caspases (caspase-3/6/7) into active “executioner” caspases. These executioner caspases then cause degradation of a variety of cellular structures, such as the cytoskeleton and nucleus, leading to apoptotic cell death.
Figure 1: The Extrinsic Pathway of Apoptosis. Source: Kashyap, Garg, & Goel (2021)
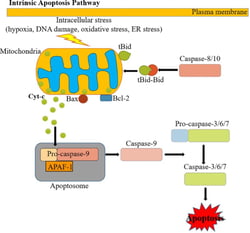 The intrinsic apoptotic pathway (mitochondria dependent) is triggered by intracellular stress or damage signals (such as ER stress, oxidative stress, DNA damage) that converge at the mitochondrial level (Figure 2). Subsequent activation of pro-apoptotic BH3-only members of the Bcl-2 family (such as Bax, Bak) neutralize the anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1, leading to disruption of mitochondrial outer membrane permeability (MOMP) so that proteins normally confined in the mitochondrial intermembrane space spread into the cytosol. These proteins include the so-called apoptogenic factors, such as cytochrome c, which plays a crucial role in activating the mitochondrial-dependent death in the cytosol. Cytochrome c binds and activates Apaf-1 as well as pro-caspase-9, forming an “apoptosome”. The process in turn activates downstream executor caspases-3, -6 and -7 for cleavage of different cellular substrates, leading to apoptotic cell death.
The intrinsic apoptotic pathway (mitochondria dependent) is triggered by intracellular stress or damage signals (such as ER stress, oxidative stress, DNA damage) that converge at the mitochondrial level (Figure 2). Subsequent activation of pro-apoptotic BH3-only members of the Bcl-2 family (such as Bax, Bak) neutralize the anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1, leading to disruption of mitochondrial outer membrane permeability (MOMP) so that proteins normally confined in the mitochondrial intermembrane space spread into the cytosol. These proteins include the so-called apoptogenic factors, such as cytochrome c, which plays a crucial role in activating the mitochondrial-dependent death in the cytosol. Cytochrome c binds and activates Apaf-1 as well as pro-caspase-9, forming an “apoptosome”. The process in turn activates downstream executor caspases-3, -6 and -7 for cleavage of different cellular substrates, leading to apoptotic cell death.
Figure 2: The Intrinsic Pathway of Apoptosis. Source: Kashyap, Garg, & Goel (2021)
An Alternative Apoptotic Pathway
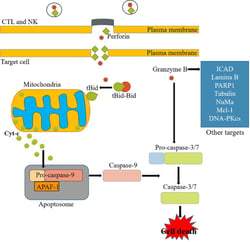 In addition to the extrinsic and intrinsic pathways, another key pathway of apoptosis involves perforins and granzymes (Figure 3). The perforin/granzyme pathway of apoptosis is the main pathway used by cytotoxic lymphocytes to kill virus-infected and transformed cells. Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells will use both the intrinsic pathway and the perforin/granzyme pathway to trigger death in the target cell. The intrinsic pathway involves the engagement and activation of certain target-cell death receptor like Fas-FasR previously discussed above. The key components, perforin and granzyme, are secreted by exocytosis by CTLs and NK cells. In a coordinated process, perforin, which is a cell membrane-disrupting protein, will create pores on the target cell membrane to facilitate the entry of granzyme, a serine protease into that cell through the pores. Subsequently, granzymes will activate caspase-9 and caspase-10, which will further activate caspase-3 and caspase-7 to trigger apoptosis.
In addition to the extrinsic and intrinsic pathways, another key pathway of apoptosis involves perforins and granzymes (Figure 3). The perforin/granzyme pathway of apoptosis is the main pathway used by cytotoxic lymphocytes to kill virus-infected and transformed cells. Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells will use both the intrinsic pathway and the perforin/granzyme pathway to trigger death in the target cell. The intrinsic pathway involves the engagement and activation of certain target-cell death receptor like Fas-FasR previously discussed above. The key components, perforin and granzyme, are secreted by exocytosis by CTLs and NK cells. In a coordinated process, perforin, which is a cell membrane-disrupting protein, will create pores on the target cell membrane to facilitate the entry of granzyme, a serine protease into that cell through the pores. Subsequently, granzymes will activate caspase-9 and caspase-10, which will further activate caspase-3 and caspase-7 to trigger apoptosis.
Figure 3: The Perforin/Granzyme Pathway of Apoptosis. Source: Kashyap, Garg, & Goel (2021)
How ABclonal Can Help
With our expertise in antibody production and characterization, ABclonal has a wide selection of antibody products to study apoptosis that includes applications such as Western blot, immunohistochemistry, and immunofluorescence. Please feel free to search our catalog or to explore a selection of our products below.
|
Target |
Cat.No. |
Product Name |
Applications |
Reactivity |
|
CHOP |
DDIT3 / CHOP Rabbit pAb |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
Bcl-2 |
Bcl-2 Rabbit mAb |
WB, IHC, IP |
Human, Mouse |
|
|
Phospho-Bcl-2-S70 Rabbit pAb |
WB, IF |
Human, Rat |
||
|
Bad |
Bad Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
|
|
Bax |
[KO Validated] Bax Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
Bak |
BAK1 Rabbit mAb |
WB |
Human |
|
|
[KO Validated] BAK1 Rabbit pAb |
WB, IF |
Human, Mouse, Rat |
||
|
p53 |
[KO Validated] p53 Rabbit mAb |
WB |
Human |
|
|
p53 Rabbit pAb |
WB, IHC, IF, ChIP |
Human, Mouse, Rat |
||
|
Puma |
PUMA Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
cIAP1/BIRC2 |
BIRC2 Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
|
|
cIAP2/BIRC3 |
BIRC3 Rabbit pAb |
WB, IF |
Human, Mouse |
|
|
XIAP/BIRC4 |
[KO Validated] XIAP Rabbit mAb |
WB |
Human, Mouse, Rat |
|
|
XIAP Rabbit pAb |
WB, IF |
Human, Mouse, Rat |
||
|
cFLIP/CFLAR |
CFLAR Rabbit pAb |
WB, IHC |
Human, Mouse, Rat |
|
|
Cytochrome C |
Cytochrome C Rabbit mAb |
WB |
Human, Mouse, Rat |
|
|
Cytochrome c Rabbit pAb |
WB, IF |
Human, Mouse, Rat |
||
|
Cytochrome c Rabbit pAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
VDAC |
VDAC1 Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
AIF |
AIF Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
|
|
[KO Validated] AIF Rabbit pAb |
WB, IHC, IF, IP |
Human, Mouse, Rat |
||
|
Endo G |
Endo G Rabbit mAb |
WB, IF |
Human, Mouse, Rat |
|
|
ENDOG Rabbit pAb |
WB, IHC |
Human, Mouse, Rat |
||
|
Smac / Diablo |
Smac / Diablo Rabbit mAb |
WB, IF |
Human, Mouse, Rat |
|
|
PARP |
[KO Validated] PARP Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
|
|
[KO Validated] Cleaved PARP p25 Rabbit mAb |
WB |
Human, Mouse |
||
|
[KO Validated] PARP1 Rabbit pAb |
WB, IHC, IF, IP, ChIP |
Human, Mouse, Rat |
References:
- Elmore, S. (2007). Apoptosis: a review of programmed cell death. Toxicologic pathology, 35(4), 495-516.
- Fuchs, Y., & Steller, H. (2011). Programmed cell death in animal development and disease. Cell, 147(4), 742-758.
- Häcker, G. (2000). The morphology of apoptosis. Cell and tissue research, 301(1), 5-17.
- Saraste, A., & Pulkki, K. (2000). Morphologic and biochemical hallmarks of apoptosis. Cardiovascular research, 45(3), 528-537.
- Kashyap, D., Garg, V. K., & Goel, N. (2021). Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Advances in Protein Chemistry and Structural Biology, 125, 73-120.
- Trapani, J. A., & Smyth, M. J. (2002). Functional significance of the perforin/granzyme cell death pathway. Nature Reviews Immunology, 2(10), 735-747.
- Hengartner, M. O. (2001). Apoptosis: corralling the corpses. Cell, 104(3), 325-328.
- Wong, R. S. (2011). Apoptosis in cancer: from pathogenesis to treatment. Journal of experimental & clinical cancer research, 30(1), 1-14.
- Igney, F. H., & Krammer, P. H. (2002). Death and anti-death: tumour resistance to apoptosis. Nature Reviews Cancer, 2(4), 277-288.
- Ashkenazi, A., & Dixit, V. M. (1998). Death receptors: signaling and modulation. science, 281(5381), 1305-1308.
- Danial, N. N., & Korsmeyer, S. J. (2004). Cell death: critical control points. Cell, 116(2), 205-219.