 You go through everyday life thanks to the intricate communication and interaction of tissue and organ functions between the trillions of cells in your body. Within those tissues, a non-cellular component exists called the extracellular matrix (ECM). Imagine a structure made of water, proteins, and polysaccharides that helps to give structural support to surrounding cells as a connective tissue. Within the ECM lies a group of enzymes named matrix metalloproteinases (MMPs). As endopeptidases, which are enzymes that break peptide bonds, the main role of MMPs is to break down collagen and other proteins in the ECM, whether in normal tissues or in promoting cancer metastasis. MMPs are divided into collagenases, gelatinases, stromelysins, matrilysins, and membrane-type (MT) MMPs, as well as some other non-classified MMPs.[1]
You go through everyday life thanks to the intricate communication and interaction of tissue and organ functions between the trillions of cells in your body. Within those tissues, a non-cellular component exists called the extracellular matrix (ECM). Imagine a structure made of water, proteins, and polysaccharides that helps to give structural support to surrounding cells as a connective tissue. Within the ECM lies a group of enzymes named matrix metalloproteinases (MMPs). As endopeptidases, which are enzymes that break peptide bonds, the main role of MMPs is to break down collagen and other proteins in the ECM, whether in normal tissues or in promoting cancer metastasis. MMPs are divided into collagenases, gelatinases, stromelysins, matrilysins, and membrane-type (MT) MMPs, as well as some other non-classified MMPs.[1]
The Structure of MMPs
MMPs have a common domain structure, which includes:
- The Pro-Peptide
- This Pro-Peptide domain is part of the cysteine switch, which prevents binding and cleavage of the substrate. This keeps the enzyme in an inactive form.
- The Catalytic Domain
- This domain contains the active site. The catalytic domain is connected to the C terminal domain by a hinge region, which is 75 amino acids long.
- The Hemopexin-like C-terminal domain
- The Hemopexin-like C-terminal domain has a four bladed β-propeller structure. This structure gives a flat surface that is theorized to be used in protein-protein interactions. This domain is missing in MMP-7, MMP-23, and MMP-26.[2]
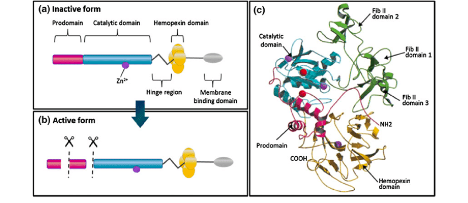
Figure 1: Structural domains of matrix metalloproteinases. (a, b) A schematic representation of the general structure of the inactive and active forms of MMPs (only the membrane-type group has the extra membrane binding domain). (b) Structure of proMMP-2. (c) The prodomain, catalytic domain, hemopexin domain and fibronectin (Fib) [1]
The Functions of MMPs
The human genome encodes 23 MMPs. MMPs are essential in many biological processes and physiological functions, while also playing a role in pathological conditions like cancer or chronic inflammatory diseases.[2] As important regulators for tissue homeostasis and immunity, unregulated MMP 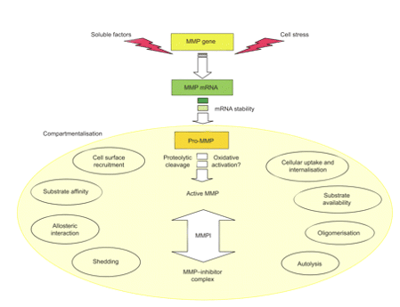 activity can lead to breakdown of homeostasis, which involves a loss of control of cellular proliferation and metabolic function, and abnormal function of programmed cell death pathways.[3]
activity can lead to breakdown of homeostasis, which involves a loss of control of cellular proliferation and metabolic function, and abnormal function of programmed cell death pathways.[3]
The catalytic activity for MMPs is controlled through gene expression with transcriptional and post-transcriptional regulation, compartmentalization, pro-enzyme activation, and enzyme inactivation.[4] Some MMPs are expressed in normal tissue like MMP-2, MMP-19, MMP-28, and several MT-MMPs, where they play a role in homeostasis. Other MMPs not normally expressed in resting tissue are performing repair processes in diseased tissues. Across varying diseases, inflammation, and tumors, we can see the importance of MMPs.
Figure 2: Matrix metalloproteinase (MMP) activity is tightly regulated at four different levels: 1) gene expression, mainly by regulating transcription and mRNA stability, 2) compartmentalization (as light yellow filled eclipse), which regulates efficiency of proteolysis through cell surface recruitment, substrate availability and protein interactions, but also influences 3) pro-enzyme activation and 4) inhibition of proteolysis.[1]
Specifically, MMP-7 is crucial for wound repair. Recent studies show that MMP-7 is essential in chemotactic recruitment of neutrophils, a white blood cell that acts as the immune system’s first line of defense, in the lung in response to infection.[2] They then contain the area using different effector mechanisms.[3] MMPs including MMP-7 are expressed in nearly every cell type in response to injury due to their very important role in healing.
How ABClonal Can Help
ABclonal offers a selection of over 80 MMP antibodies with different applications and reactivities. A selection of our MMP antibody products is shown below.
|
Target |
Cat No. |
Product Name |
|
MT1-MMP |
A0067 |
MMP14/MT1-MMP Rabbit mAb |
|
MMP1 |
A1191
|
MMP1 Rabbit pAb |
|
MMP2 |
A19080
|
[KO Validated] MMP2 Rabbit mAb |
A6247
|
MMP2 Rabbit pAb |
|
|
MMP3 |
A11418
|
MMP3 Rabbit mAb |
A1202
|
MMP3 Rabbit pAb |
|
|
MMP7 |
A0695 |
MMP7 Rabbit pAb |
|
MMP8 |
A1963
|
MMP8 Rabbit pAb |
|
MMP9 |
A2095
|
MMP9 Rabbit pAb |
A0289 |
MMP9 Rabbit pAb |
|
|
MMP10 |
A3033 |
MMP10 Rabbit pAb |
|
MMP11 |
A11088 |
|
|
MMP12 |
A11017 |
|
|
A1709 |
|
|
|
MMP13 |
A11148 |
|
|
A1606 |
|
|
|
MMP16 |
A10409 |
|
|
MMP17 |
A3030 |
|
|
MMP25 |
A15886 |
|
References
- J Enzyme Inhib Med Chem. 2016;31(sup1):177-183.
- “Matrix Metalloproteinase,” Wikipedia (Wikimedia Foundation, December 10, 2021).
- Javaid, Mohammad Ahmad & Abdallah, Mohamed-Nur & Ahmed, Ahad & Sheikh, Zeeshan. (2013). Matrix metalloproteinases and their pathological upregulation in multiple sclerosis: An overview. Acta neurologica Belgica. 113. 10.1007/s13760-013-0239-x.
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer 2007; 7: 800–808
- “Tissue Homeostasis.” Royal Society of Chemistry. Accessed July 6, 2022.
- Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26(8):587-596. doi:10.1016/j.matbio.2007.07.001
- Löffek, S., O. Schilling, and C-W. Franzke. “Biological Role of Matrix Metalloproteinases: A Critical Balance.” European Respiratory Society. European Respiratory Society, July 1, 2011.
- Chen P, Abacherli LE, Nadler ST, et al. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS One 2009; 4: e6565.
- SenGupta S, Hein LE and Parent CA (2021) The Recruitment of Neutrophils to the Tumor Microenvironment Is Regulated by Multiple Mediators. Front. Immunol. 12:734188. doi: 10.3389/fimmu.2021.734188

-1.png)