Previously, I listed some of my favorite educational YouTube channels, which includes the Kurzgesagt channel with its fun animations and soothing narration of scientific concepts, including many topics in biology and biological functions. As part of my algorithm, this one popped up in my feed recently:
The video is mostly about translation of genetic code into polypeptide synthesis, and if you listen to around the 3:45 mark, you may hear the narrator suggest 21 amino acids. Like me, you may stop in your tracks and think, "Twenty-one? I thought there were only twenty!" But that is where our story begins as I went down a rabbit hole of literature research to learn more about the essential element, selenium, and its incorporation into the amino acid selenocysteine.
Whither Selenium?
 Admittedly, the only real knowledge I have about the element selenium is that it is on the periodic table of elements, in the same group as oxygen and sulfur, and that it is found in shampoo. It also happens to be a major plot point in the so-bad-it's-good movie Evolution, where selenium was needed to destroy the invasive species threatening our planet so they dumped hundreds of gallons of shampoo on them and saved the world. But besides being an active ingredient in shampoos to relieve itching and flaking scalp while killing the fungal infections that can cause dandruff, selenium is an essential trace element that is incorporated into many enzymes and proteins useful in protecting the body from oxidative stress and damage. According to the resource at Harvard, the population of the United States generally should get enough selenium through their foods since the soil is naturally rich in the element, and we can source selenium from common foods including poultry, beef, and whole wheat bread.
Admittedly, the only real knowledge I have about the element selenium is that it is on the periodic table of elements, in the same group as oxygen and sulfur, and that it is found in shampoo. It also happens to be a major plot point in the so-bad-it's-good movie Evolution, where selenium was needed to destroy the invasive species threatening our planet so they dumped hundreds of gallons of shampoo on them and saved the world. But besides being an active ingredient in shampoos to relieve itching and flaking scalp while killing the fungal infections that can cause dandruff, selenium is an essential trace element that is incorporated into many enzymes and proteins useful in protecting the body from oxidative stress and damage. According to the resource at Harvard, the population of the United States generally should get enough selenium through their foods since the soil is naturally rich in the element, and we can source selenium from common foods including poultry, beef, and whole wheat bread.
While selenium itself was isolated in the 19th Century, it wasn't until the 1950s that it was first considered to be an essential trace element, and later on, a critical antioxidant that could protect cell membranes against oxidative damage. 1 Fortuitously, at this writing it is Women's History Month, and the existence of the amino acid selenocysteine was discovered by the research group led by Thressa Stadtman. Selenocysteine is similar to the amino acid we already know, cysteine, except that the sulfur atom has been replaced with selenium. Proteins that incorporate selenocysteine (abbreviated Sec) are known as selenoproteins, of which more are being discovered each day in human biology. This is different from proteins that merely have a selenium as a co-factor, such as the glutathione peroxidases (the GPX family, of which GPX4 is a member), and Sec is unlike post-translationally modified amino acids such as arginine-to-citrulline or the various phosphorylations of serine, threonine, and tyrosine, among others, since Sec is incorporated directly into a growing polypeptide chain by a ribosome.
"Stop" is Just a Suggestion
Usually, when you consider the classical protein synthesis pathway, translation stops when the ribosome encounters the three stop codons, which are UGA, UAG, or UAA. It appears that in some cases, if the UGA codon is within the coding sequence of the message, it can be redefined as a signal for selenocysteine, even in human proteins, when there are additional genetic cues that direct the incorporation of Sec in the protein. 2 There is plenty of debate over why some organisms can use this mechanism to incorporate Sec while other organisms do not, another quirk in our long evolutionary history.
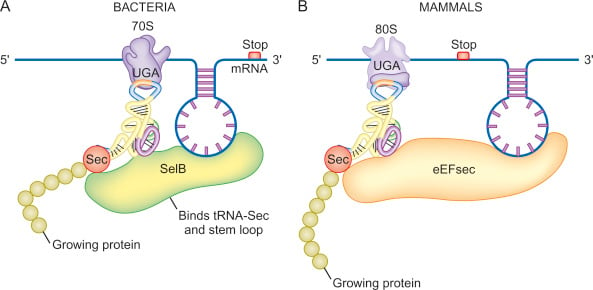
The insertion of Sec into proteins in A) bacteria versus B) mammals, from ScienceDirect.
Many selenoproteins use Sec primarily for their catalytic activity, as substituting Sec for cysteine sharply reduces their function. 1 One theory is that the selenium in Sec can resist overoxidization, since selenoproteins can reverse the oxidizing of Sec much more easily than similar oxidation of cysteines. A large proportion of the research in Sec, incorporation into selenoproteins, and the function of these proteins involves understanding the evolutionary advantages of these comparatively rare proteins using a trace element in the context of their obvious benefits to human health.
As of 2022, most scientists agree there are at least 25 selenoproteins and some additional Sec machinery proteins used to incorporate Sec into these proteins using one Sec-specific transfer RNA. 3 Due to the antioxidant usage of selenium in the body, it is not surprising to find selenoproteins in close proximity to other proteins involved in reducing oxidative stress, such as the glutathione peroxidases (GPX4), fatty acid metabolic enzymes like the stearoyl-CoA desaturase (SCD), thioredoxin reductases (TXNRD1), and peroxiredoxins (PRDX6). 3
The Role of Selenium and Selenoproteins in Health and Disease
 We introduced selenium above as an essential trace element, with antioxidant properties that decompose peroxides and reduce DNA and tissue damage. Getting enough selenium into our systems is important as it can help reduce the risk of cancer, thyroid complications, and cardiovascular diseases due primarily to these proteins being able to reduce oxidative stress and the inflammation and other damage resulting from oxidation.
We introduced selenium above as an essential trace element, with antioxidant properties that decompose peroxides and reduce DNA and tissue damage. Getting enough selenium into our systems is important as it can help reduce the risk of cancer, thyroid complications, and cardiovascular diseases due primarily to these proteins being able to reduce oxidative stress and the inflammation and other damage resulting from oxidation.
The primary stores of selenium reside in the thyroid gland, which uses various selenoproteins to regulate thyroid function. Without adequate supplies of selenium and by extension the Sec required to produce the crucial selenoproteins, this could result in autoimmunity that targets the thyroid gland, leading to conditions such as Hashimoto's disease and Graves' disease. Research suggests that selenium and selenoproteins can be commandeered by cancer cells to protect the tumor against oxidative damage and against ferroptosis, which requires GPX4 function and plenty of selenium intake, which might make these proteins (and perhaps selenium itself) an intriguing target against certain types of cancers. 4
While it is unclear whether selenium supplements can reduce disease risk, it is known that not enough selenium can cause various cardiac and bone diseases, with unpleasant symptoms including nausea, neurological issues, and fatigue, whereas like everything else, too much selenium could lead to toxicity. The recommendation from Harvard's resource is to get at least 55 micrograms daily, while the upper limit is around 400 micrograms daily, although there's at least some evidence that, like with zinc, selenium can help boost immune function. Although it is unlikely you will need to ever use gallons of Head and Shoulders to defeat monsters that are susceptible to selenium, know that at least a little bit of selenium can go a long way in keeping you happy and healthy!
References
- Gonzalez-Flores et al. (2013) "The Molecular Biology of Selenocysteine." Biomol Concepts 4(4):349-365.
- Copeland PR (2005) "Making sense of nonsense: the evolution of selenocysteine usage in proteins." Genome Biology 6:221 (Epub).
- Santesmasses D & Gladyshev VN (2022) "Selenocysteine Machinery Primarily Supports TXNRD1 and GPX4 Functions and Together They Are Functionally Linked with SCD and PRDX6." Biomolecules 12(8):1049 (Epub).
- Shimada et al. (2022) "Metabolism of Selenium, Selenocysteine, and Selenoproteins in Ferroptosis in Solid Tumor Cancers." Biomolecules 12(11):1581 (Epub).