The tumor is an abnormal tissue mass formed when cells divide and grow excessively within the body. Tumors can be benign (not cancerous) or malignant (cancerous). Benign tumors may become larger but do not spread to nearby tissue or other parts of the body. Malignant tumors, on the other hand, can spread nearby to tissue and can also be transmitted to other parts of the body through the blood and or lymphatic system.1 But we are no strangers to tumors and how the develop.
On the other hand, many of us aren’t as familiar with a tumor’s environment. Tumor progression is profoundly affected by the subtle interaction of tumor cells with immune and non-immune cells within their environment. In particular, the interactions with the immune cell component of a tumor are fundamental in determining whether primary tumors are eradicated, metastasized, or established by dormant micro metastases.3 The environment that a tumor grows in is also much more complex than one would think because of its highly variable cell composition, large number of proteins, and structures involved in tumor formation.
This being said, tumor microenvironment includes:
• Heterogeneous populations of cancer cells
• A variety of resident and osmotic host cells
• Secretion factors
• Extracellular matrix proteins
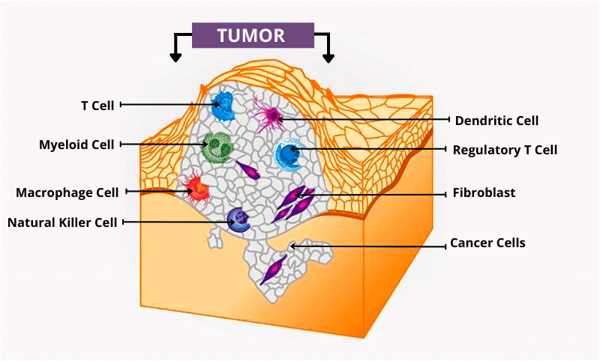
Figure 1 source: National Cancer Institute. (2020, January 15). What is the tumor microenvironment? Image details. NCI Visuals Online. Retrieved from https://visualsonline.cancer.gov/details.cfm?imageid=12496.
Key Role in Cancer Development
The extracellular matrix (ECM) is a major structural component of the tumor microenvironment.5 While a tumor microenvironment provides all the nutrients (oxygen, glucose, etc.) needed for a tumor to grow, the ECM contains all the cytokines, growth factors, and hormones secreted by stromal and tumor cells. As time goes on, and the immune and non-immune cells of the tumor microenvironment interact with each other and can allow the cancer to grow. Then, the immune microenvironment helps cancer cells select dominant cells, allowing tumors to progress and or grow. The ECM can aid tumor development in the following ways:
- Affect tumors through extracellular secretion.
- Change the phenotypic type of somatic cell or tumor cells.
- Help tumors get rid of immune monitoring.
- Provide a low-oxygen or acidic environment in which tumor cells have a more significant survival advantage than normal cells.
Methods of Tumor microenvironment Phenotyping
The tumor immune microenvironment phenotyping (TME) method utilizes multiplex immunohistochemistry (mIHC) and immunofluorescence (IF) to explore specific immune cells. IF is a common laboratory technique used to determine the location of an antigen in tissues by its reaction with an antibody labeled with a fluorescent dye. With this fluorescent dye attached, the target molecule can be depicted in a way that allows researchers to study the distribution within the sample. Furthermore, mIHC is a form of immunostaining that uses enzymes or fluorescent-related antibodies to identify antigens in tissue parts. This technique can be used to track multiple markings simultaneously without the need for laborious dye cycling procedures.4 Overall, it has been found that the TME phenotyping methods discussed previously can contribute to cancer patients' clinical prognosis and efficacy prediction.
Improving Cancer Treatment
It is important to understand TME and the different immune cells in it to leverage the immunotherapy and the developing new treatments, as many cancer types often have similar tumor microenvironments. Scientists can use different predefined cell type markers to describe the state and spatial arrangement of tumor cells and different types of osmosis strobe cells, analyze TME to discover new cell phenotypes and disease mechanisms, develop targeted therapies, study drug efficacy, and predict the prognosis for cancer treatment.
Current Developments
In recent years, many studies have shown that significant epigenetic changes lead to abnormal gene expression in tumor microenvironment cells. The characteristics of the gene expression from tumor stroma can predict clinical outcomes. Given these recent findings, researchers are increasingly interested in the cancer microenvironment as a prognostic factor and potential therapeutic target. New therapies targeting matrix components are being developed.
Monoclonal antibody (mAb) therapy has only recently become one of the main ways to treat cancer.6 Monoclonal antibodies target tumor cell-specific or over-expressed antigens that can cause tumor cell death. When a mAb binds to its target growth factor receptor and manipulates its activation state or blocks ligand binding, the signals that promote tumor growth and survival will be disturbed.
According to statistics, many monoclonal antibodies have been approved for oncology, autoimmune diseases, chronic diseases, and more diseases. More than 80 antibody therapies have received regulatory approvals in Europe and the United States.7 In 2017 alone, global sales of therapeutic antibodies exceeded $100 billion US dollar.8
How ABclonal can Help
Scientists can effectively and accurately characterize the tumor microenvironment using antibodies through IF and mIHC to understand how different cell types in the tumor microenvironment interact and communicate with each other through signaling networks. Once researchers understand these signaling networks, they will be able to develop different ways to block these signals, thereby preventing tumors from growing. They can also utilize mAb therapies to target tumors. At ABclonal Technology, we offer multiple antibodies for tumor-microenvironment phenotyping.
Featured Antibodies from ABclonal for Tumor microenvironment Phenotyping
|
Category |
Target |
Cat. No. |
Product Name |
Applications |
Reactivity |
|
Cancer cell related |
E-Cadherin |
E-Cadherin mouse mAb |
WB,IHC,FC |
Human,Mouse |
|
|
EpCAM |
EpCAM Rabbit mAb |
WB,IHC,IF |
Human,Mouse,Rat |
||
|
Cytokeratin 8 |
[KO Validated] Cytokeratin 8 Rabbit pAb |
WB, IHC, IF |
Human, Mouse |
||
|
Cytokeratin 14 |
Cytokeratin 14 (KRT14) Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
||
|
Cytokeratin 18 |
Cytokeratin 18 (KRT18) Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
Cytokeratin 19 |
Cytokeratin 19 (KRT19) Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
||
|
Claudin 1 |
Claudin 1 Rabbit pAb |
WB, IHC |
Human, Mouse, Rat |
||
|
CEACAM1 |
CEACAM1 Rabbit mAb |
WB,IHC |
Human,Rat |
||
|
CEACAM5 |
CEACAM5 Mouse mAb |
WB,IHC |
Human |
||
|
MUC1 |
MUC1 Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
||
|
CD13/ANPEP |
CD13/ANPEP Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
CD34 |
CD34 Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
||
|
CD38 |
CD38 Rabbit mAb |
IF, FC |
Human |
||
|
CD82 |
CD82 Rabbit mAb |
WB, IHC, IF |
Human, Mouse |
||
|
CD83 |
CD83 Rabbit mAb |
WB, IF |
Human, Mouse |
||
|
CD90/Thy1 |
CD90/Thy1 Rabbit mAb |
WB,IHC |
Human,Rat |
||
|
CD105/Endoglin |
CD105/Endoglin Rabbit mAb |
WB, IHC |
Human |
||
|
CD117/c-Kit |
CD117/c-Kit Rabbit pAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
CD138/Syndecan-1 |
CD138/Syndecan-1 Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
AFP |
Alpha-Fetoprotein (AFP) Mouse mAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
ALDH1A1 |
ALDH1A1 Rabbit mAb |
WB,IF |
Human, Mouse, Rat |
||
|
CD44 |
[KO Validated] CD44 Rabbit mAb |
WB,IHC |
Human |
||
|
CD133 |
[KO Validated] CD133 Rabbit pAb |
WB,IF,IP |
Human,Mouse,Rat |
||
|
SOX2 |
[KO Validated] SOX2 Rabbit mAb |
WB |
Human,Mouse,Rat |
||
|
Fibroblasts related |
α-SMA |
α-Smooth Muscle Actin (ACTA2) Rabbit mAb |
WB,IHC,IF |
Human,Mouse,Rat |
|
|
FSP1/S100A4 |
FSP1/S100A4 Rabbit mAb |
WB,IHC,IF |
Human,Mouse,Rat |
||
|
Fibronectin |
Fibronectin Rabbit pAb |
WB, IHC, IF |
Human, Mouse |
||
|
Vimentin |
[KO Validated] Vimentin Rabbit mAb |
WB, IHC, IF, IP |
Human, Mouse, Rat |
||
|
FGFR3 |
FGFR3 Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
FGFR4 |
FGFR4 Rabbit mAb |
WB, IHC |
Human, Mouse |
||
|
PDGFRB |
PDGF Receptor beta Rabbit mAb |
WB, IF |
Human, Mouse, Rat |
||
|
T cell related |
CD3D |
CD3D Rabbit mAb |
WB, IF |
Human, Mouse, Rat |
|
|
CD3E |
CD3 epsilon Rabbit mAb |
WB, IHC |
Human |
||
|
CD3E/G |
CD3(epsilon+gamma)/CD3E+CD3G Rabbit mAb |
WB, IF, FC, ELISA |
Human |
||
|
CD4 |
CD4 Rabbit mAb |
WB, IHC |
Human |
||
|
CD8 |
CD8A Rabbit mAb |
WB, IHC |
Human |
||
|
B cell related |
CD19 |
CD19 Rabbit mAb |
WB, IHC |
Human |
|
|
CD20 |
CD20 Rabbit mAb |
WB, IHC, IF |
Human |
||
|
CD79a |
CD79a Rabbit mAb |
WB, IHC |
Human |
||
|
CD79b |
CD79B Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
NK cell marker |
NCAM1/CD56 |
NCAM1 / CD56 Rabbit pAb |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
Cytotoxic molecules |
Granzyme B |
Granzyme B Rabbit pAb |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
Perforin |
Perforin Rabbit pAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
Endothelial cell marker |
CD31 |
CD31/PECAM1 Rabbit mAb |
WB, IHC |
Human |
|
|
CD31 |
CD31/PECAM1 Rabbit mAb |
WB, IF |
Human, Mouse |
||
|
CD31 |
CD31/PECAM1 Rabbit mAb |
WB, IHC |
Human |
||
|
Immune checkpoints |
CD27 |
CD27 Rabbit mAb |
WB,IF |
Human,Rat |
|
|
CD28 |
CD28 Rabbit mAb |
FC, ELISA |
Human |
||
|
CD40 |
CD40 Rabbit mAb |
WB, IF, FC, ELISA |
Human |
||
|
Arginase 1 |
Arginase 1 (ARG1) Rabbit mAb |
WB, IHC, IF |
Human, Mouse, Rat |
||
|
CD112 |
Nectin 2/CD112 Rabbit mAb |
WB, IHC |
Human, Mouse, Rat |
||
|
CD274/PD-L1 |
[KO Validated] PD-L1 Rabbit mAb |
WB, IHC |
Human, Rat |
||
|
CD274/PD-L1 |
PD-L1 Rabbit mAb |
WB, IHC |
Human |
||
|
CD274/PD-L1 |
PD-L1 Rabbit mAb |
WB, FC, ELISA |
Human |
References:
Ham, S., Lima, L. G., Lek, E., & Möller, A. (2020, June 23). The impact of the cancer microenvironment on macrophage phenotypes. Frontiers. Retrieved from https://www.frontiersin.org/articles/10.3389/fimmu.2020.01308/full.Jin, MZ., Jin, WL. The updated landscape of tumor microenvironment and drug repurposing. Sig Transduct Target Ther 5, 166 (2020). https://doi.org/10.1038/s41392-020-00280-x
Barry, S., Carlsen, E., Marques, P. et al. Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene 38, 5381–5395 (2019). https://doi.org/10.1038/s41388-019-0779-5
Kevin Mayer. (2021, April 9). Immune cell profiles reveal cancer's leading indicators. Immune Cell Profiles Reveal Cancer’s Leading Indicators. Retrieved from https://www.genengnews.com/topics/cancer/immune-cell-profiles-reveal-cancers-leading-indicators/.
Wang, M., Zhao, J., Zhang, L., Wei, F., Lian, Y., Wu, Y., Gong, Z., Zhang, S., Zhou, J., Cao, K., Li, X., Xiong, W., Li, G., Zeng, Z., & Guo, C. (2017). Role of tumor microenvironment in tumorigenesis. Journal of Cancer, 8(5), 761–773. https://doi.org/10.7150/jca.17648
Cruz, E., & Kayser, V. (2019). Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics : targets & therapy, 13, 33–51. https://doi.org/10.2147/BTT.S166310
Zahavi, D., & Weiner, L. (2020). Monoclonal Antibodies in Cancer Therapy. Antibodies, 9(3), 34. https://doi.org/10.3390/antib9030034
Walsh, G. Biopharmaceutical benchmarks 2018. Nat Biotechnol 36, 1136–1145 (2018). https://doi.org/10.1038/nbt.4305