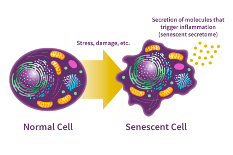
 Reaching the golden years doesn’t always feel so golden. As we age, disease, injury, and other stress factors from the environment will damage our bodies' cells. Most cells may be able to repair that damage, while our immune system usually clears those damaged cells through a process called apoptosis.[1] However, if cellular repair and clearance is not effective, the residual damaged cells will further weaken the immune system and deteriorate other biological processes. Is there a possibility that we can avoid this cellular damage and improve the health of older people? A cellular state known as senescence might hold the key to this question.[1, 2] During senescence, the damaged cells irreversibly stop dividing and resist being removed. [3] Researchers have shown that determining senescence biomarkers could lead to new therapies for the inflammatory disease caused by senescence in older people.[4]
Reaching the golden years doesn’t always feel so golden. As we age, disease, injury, and other stress factors from the environment will damage our bodies' cells. Most cells may be able to repair that damage, while our immune system usually clears those damaged cells through a process called apoptosis.[1] However, if cellular repair and clearance is not effective, the residual damaged cells will further weaken the immune system and deteriorate other biological processes. Is there a possibility that we can avoid this cellular damage and improve the health of older people? A cellular state known as senescence might hold the key to this question.[1, 2] During senescence, the damaged cells irreversibly stop dividing and resist being removed. [3] Researchers have shown that determining senescence biomarkers could lead to new therapies for the inflammatory disease caused by senescence in older people.[4]
What is Cellular Senescence?
 Cellular senescence refers to a state of stable cell cycle arrest in which proliferating cells become resistant to growth-promoting stimuli, typically in response to DNA damage. The phenomenon was first described by Leonard Hayflick upon the observation that human fetal fibroblasts eventually stopped dividing, but remained viable and metabolically active after prolonged time in culture.[3] More recently, cell senescence was identified as a response opposing oncogenic transformation, as research has shown that cells carrying an activated oncogene either die through apoptosis or enter the characteristic stable cell cycle arrest that defines cell senescence. [5]
Cellular senescence refers to a state of stable cell cycle arrest in which proliferating cells become resistant to growth-promoting stimuli, typically in response to DNA damage. The phenomenon was first described by Leonard Hayflick upon the observation that human fetal fibroblasts eventually stopped dividing, but remained viable and metabolically active after prolonged time in culture.[3] More recently, cell senescence was identified as a response opposing oncogenic transformation, as research has shown that cells carrying an activated oncogene either die through apoptosis or enter the characteristic stable cell cycle arrest that defines cell senescence. [5]
Biomarkers for Senescence
Modern science has profiled specific biomarkers that facilitate the unequivocal detection and quantification of senescent cells in vitro and in vivo, which consists of the elevated senescence-associated β-galactosidase (SA-β-gal) activity [6], cell cycle regulators including p16INK4a, p21CIP1, and p53[7], DNA damage markers like Phospho-gamma-H2A.X[8] and Phospho-ATM [9], global heterochromatin loss characterized by H3K9me3 and H3K27me3[10], senescence-induced lamin B1 downregulation [11], negativity of proliferation marker Ki-67[12], senescence-associated secretory phenotype (SASP)[13] containing HMGB1, MMPs, IL-6, IL-8, and other characteristic factors.
How ABclonal Can Help
ABclonal offers a diverse selection of antibodies to facilitate cell senescence research. Please see a selection of markers below, or search our vast catalog for your favorite target.
|
Target |
Cat.No. |
Product Name |
Applications |
Reactivity |
|
p16 INK4A |
A11651 |
WB, IHC, IF |
Human |
|
|
p21 Waf1/Cip1 |
A19094 |
WB, IHC |
Human |
|
|
Phospho-gamma-H2A.X |
AP0687 |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
H3K9me3 |
A2360 |
WB, IHC, IF, IP, ChIP, ChIP-seq |
Human, Mouse, Rat, Other (Wide Range) |
|
|
H3K27me3 |
A2363 |
WB, IHC, IF, IP, ChIP, ChIP-seq |
Human, Mouse, Rat, Other (Wide Range) |
|
|
ATM |
A19650 |
WB, IHC |
Human, Mouse, Rat |
|
|
Phospho-ATM |
AP1030 |
WB |
Human |
|
|
p53 |
A19585 |
WB |
Human |
|
|
A0263 |
WB, IHC, IF, ChIP |
Human, Rat |
||
|
Phospho-p53 |
AP0083 |
WB, IF, IP |
Human, Mouse, Rat |
|
|
Rb |
A3618 |
WB |
Human |
|
|
Phospho-Rb |
AP0117 |
WB, IHC |
Human, Mouse, Rat |
|
|
Ki67 |
A11390 |
WB, IF |
Human, Mouse, Rat |
|
|
Lamin B1 |
A4373 |
WB, IF |
Human, Mouse, Rat |
|
|
IL1 beta |
A16288 |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
IL6 |
A11115 |
WB, IHC |
Human, Mouse, Rat |
|
|
TNF-α |
A11534 |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
IL8 |
A2541 |
WB, IHC |
Human |
|
|
HMGB1 |
A19529 |
WB, IHC, IF |
Human, Mouse, Rat |
|
|
MMP2 |
A6247 |
WB, IHC |
Human, Mouse, Rat |
|
|
MMP3 |
A11418 |
WB, IHC |
Human, Mouse, Rat |
|
|
MMP9 |
A11147 |
WB, IHC |
Human, Mouse, Rat |
References
- "Does Cellular Senescence Hold Secrets For Healthier Aging?". National Institute On Aging, 2022, https://www.nia.nih.gov/news/does-cellular-senescence-hold-secrets-healthier-aging.
- Gong, Longyuan et al. "Senescence - Latest Research And News | Nature". Nature.Com, 2022, https://www.nature.com/subjects/senescence.
- "Overview Of Cellular Senescence And Aging | Cell Signaling Technology". Cell Signaling Technology, 2022, https://www.cellsignal.com/science-resources/overview-of-cellular-senescence.
- "How Studying Cellular Senescence Can Help Researchers Learn To Delay Aging". Yale School Of Medicine, 2022, https://medicine.yale.edu/news-article/how-studying-cellular-senescence-can-help-researchers-learn-to-delay-aging/.
- Da Silva-Álvarez, S., and M. Collado. "Cellular Senescence". Encyclopedia Of Cell Biology, 2016, pp. 511-517. Elsevier, https://doi.org/10.1016/b978-0-12-394447-4.30066-9.
- Debacq-Chainaux et al. (2009) “Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo.” Nature Protocols 4:1798-1806.
- Collado M & Serrano M (2010) “Senescence in tumours: evidence from mice and humans.” Nature Reviews Cancer 10(1):51-57.
- di Fagagna et al. (2003) “A DNA damage checkpoint response in telomere-initiated senescence.” Nature 426:194-198.
- Zhou L (2007) “Single- and double-stranded DNA: building a trigger of ATR-mediated DNA damage response.” Genes & Development 21:879-885.
- Tsurumi A & Li WX (2012) “Global heterochromatin loss: a unifying theory of aging?” Epigenetics 7(7):680-688.
- Shimi et al. (2011) “The role of nuclear lamin B1 in cell proliferation and senescence.” Genes & Development 25:2579-2593.
- Lawless et al. (2010) “Quantitative assessment of markers for cell senescence.” Exp Gerontol 45(10):772-778.
- Acosta et al. (2008) “Chemokine signaling via the CXCR2 receptor reinforces senescence.” Cell 133(6):1006-1018.