In a previous article, we explored the differences between rabbit and mouse antibodies as well as the biology behind rabbit antibody superiority. But after choosing the host, the type of technology used to produce the antibody is important too. Here, we explore some of the rabbit monoclonal antibody technologies available in the current market.
Hybridoma fusion technology
Since the development of hybridoma fusion technology in 1975 by Georges Kohler and Cesar Milstein, and its application to the development of mouse monoclonal antibodies, it has had a major impact on modern biomedical research. This is the oldest monoclonal antibody preparation technology: to immortalize antibody-secreting B cells by merging B lymphocytes and myeloma cells in immunized animals. The hybridoma cell line secreting the monoclonal antibody is then selected using limited dilution cloning. The monoclonal antibody is produced on a large scale by amplifying the culture of the cell strain.
Hybridoma cell fusion technology is the most widely used technology in the development and preparation of mouse monoclonal antibodies. Since the 1980s, researchers have been working on the development of rabbit monoclonal antibodies based on hybridoma technology. However, due to the lack of a better rabbit myeloma cell line as a fusion cell strain, the progress has not been smooth. Therefore, early researchers also tried to create heterologous fusion hybridoma cell lines using mouse murine myeloma cell lines and rabbit B lymphocytes. However, this method is not very effective due to low fusion efficiency, genomic instability and defects in rabbit antibody heavy chain light chain pairing.
Phage display library technology
In 1985, George Smith et al. found that the pIII gene (encodes the phage mycelial protein) could be altered by genetic engineering to express different peptides or protein sequences without affecting the activity and infection function of phage. The use of phage display technology to screen monoclonal antibodies then began in the early 1990s, and the scFv and Fab fragments of the target antibody were successfully screened by constructing a phage library encoding the antibody gene. Currently, phage display technology is still the main method used to screen scFv and Fab fragments.
However, phage display technology still has the following drawbacks:
- It relies on the expression system of phage and prokaryotic bacteria, so that the heavy and light chains of the antibody do not necessarily have the correct transcription, translation, post-translational modification, folding, and assembly in the system.
- The pairing of heavy and light chains in the antibodies produced using phage display is a random, artificial pairing. Much like other display technologies (such as yeast system display technology), the pairing cannot truly reflect chain pairing processes in natural, in vivo environments.
- Screening of monoclonal antibodies by phage display technology is also very dependent on library capacity. Only by screening a large-diversity antibody library, is there a probability of obtaining high-affinity rabbit monoclonal antibodies.
- Compared to hybridoma fusion, the technical requirements are much higher for phage display.
Proteomics and NGS combined technology
NG-XMTTM technology was developed by Cell Signaling Technology in 2012. It combines next-generation sequencing (NGS) with proteomics. The antibodies are obtained from the peripheral blood of immunized animals using the protein antigen and the amino acid sequence information is gained using mass spectrometry.
However, there are still some technical problems unsolved in the method, and it has not been extended to large-scale commercial application. For example, the cognate heavy/light chain pairing information cannot be gained using mass spectrometry and NGS. In addition, after obtaining the heavy and light chain gene information encoding the antibody, these techniques need to be verified by recombinant expression in vitro, and the cost can quickly stack up.
Single-cell screening and cloning technology
Phage display libraries to screen rabbit monoclonal antibodies has some of the above-mentioned disadvantages, and traditional hybridoma cell technology still has low fusion efficiency, low hybridoma cell line stability, and it is still protected under patents. Therefore, since the early 2000s, researchers have been developing a new generation of rabbit monoclonal antibody development technology independent of hybridoma fusion technology and phage display technology.
Rabbit monoclonal antibody production by this method mainly entails of selecting specific B lymphocytes by conducting multicolor flow cytometry using B cell surface markers and then cloning the single B cell heavy and light chain genes in vitro. The advantages of this method are twofold. First, like hybridoma fusion, the heavy/light chain pairing of the obtained antibody completely reflects pairings under natural conditions. Second, the B cells can be isolated from the peripheral blood lymphocytes, hence screening procedures can be performed without killing the animal.
Currently, the most common method used to obtain the corresponding monoclonal antibody heavy and light chain genes from a single B cell is direct PCR from a single B cell.
In general, single-cell screening and cloning have the following disadvantages in commercial applications:
- Single-cell PCR is highly technical and requires very specific environmental conditions. In addition, more cycles (generally > 50) or multiple rounds of PCR are required to effectively amplify the heavy and light chain genes of the antibody. Hence, the probability of PCR-mediated mutations in the target gene increases, and functional antibodies may be lost.
- Like NGS, the heavy/light chain information obtained needs to be verified by recombinant expression in vitro.
- Since commercial antibodies against rabbit B lymphocyte surface markers are still scarce, the purity of rabbit B lymphocytes obtained by screening is limited, and the proportion of positive clones obtained is lower.
- The cloning of antibody genes from single cells and the in vitro recombinant expression verification is time-consuming and results in heavy workloads. Therefore, this method is not suitable for large-throughput screening.
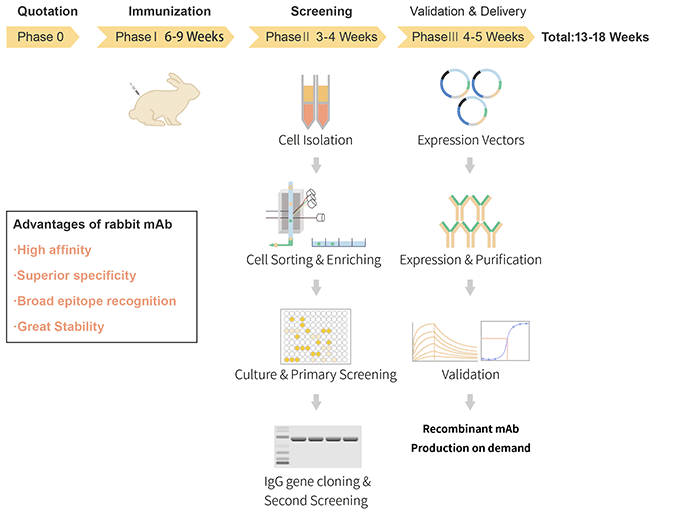
At ABclonal Technology, an improved version of the single-cell screening and cloning technology is used. In this technology, an optimized rabbit culture medium is used to culture the individual rabbit B lymphocytes obtained by sorting. The B cells are stimulated to proliferate in vitro and secrete a sufficient amount of antibody IgG for primary screening. It is well known that the B lymphocytes obtained by sorting are primary cells, and the cultivation of primary cells has always been a difficult problem in cell biology research, the rabbit B cell and plasma cell cloning technology overcomes this issue. Find out more through our website or by contacting us.
For further reading and more helpful tips for your research and experiments, check out our curated list of blogs here to learn more about various lab techniques and experimental troubleshooting.
References:
Babcook, J.S., Leslie, K.B., Olsen, O.A., Salmon, R.A. and Schrader, J.W., 1996. A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proceedings of the National Academy of Sciences, 93(15), pp.7843-7848.
Cheung, W.C., Beausoleil, S.A., Zhang, X., Sato, S., Schieferl, S.M., Wieler, J.S., Beaudet, J.G., Ramenani, R.K., Popova, L., Comb, M.J. and Rush, J., 2012. A proteomics approach for the identification and cloning of monoclonal antibodies from serum. Nature biotechnology, 30(5), p.447.
Clackson, T., Hoogenboom, H.R., Griffiths, A.D. and Winter, G., 1991. Making antibody fragments using phage display libraries. Nature, 352(6336), p.624.
Friedman, M.L., Tunyaplin, C., Zhai, S.K. and Knight, K.L., 1994. Neonatal VH, D, and JH gene usage in rabbit B lineage cells. The Journal of Immunology, 152(2), pp.632-641.
Lightwood, D.J., Carrington, B., Henry, A.J., McKnight, A.J., Crook, K., Cromie, K. and Lawson, A.D., 2006. Antibody generation through B cell panning on antigen followed by in situ culture and direct RT-PCR on cells harvested en masse from antigen-positive wells. Journal of immunological methods, 316(1-2), pp.133-143.
Mage, R.G., 1998. Diversification of rabbit VH genes by gene‐conversion‐like and hypermutation mechanisms. Immunological reviews, 162(1), pp.49-54.
Mage, R.G., Lanning, D. and Knight, K.L., 2006. B cell and antibody repertoire development in rabbits: the requirement of gut-associated lymphoid tissues. Developmental & Comparative Immunology, 30(1-2), pp.137-153.
Raybould, T.J. and Takahashi, M., 1988. Production of stable rabbit-mouse hybridomas that secrete rabbit mAb of defined specificity. Science, 240(4860), pp.1788-1790.
Van Rhijn, I., Godfrey, D.I., Rossjohn, J. and Moody, D.B., 2015. Lipid and small-molecule display by CD1 and MR1. Nature Reviews Immunology, 15(10), p.643.
Weber, J., Peng, H. and Rader, C., 2017. From rabbit antibody repertoires to rabbit monoclonal antibodies. Experimental & molecular medicine, 49(3), p.e305.
Yam, P.C. and Knight, K.L., 2014. Generation of rabbit monoclonal antibodies. In Monoclonal Antibodies (pp. 71-79). Humana Press, Totowa, NJ.
Zhu, X., Boonthum, A., Zhai, S.K. and Knight, K.L., 1999. B lymphocyte selection and age-related changes in VH gene usage in mutant Alicia rabbits. The Journal of Immunology, 163(6), pp.3313-3320.
Zhu, W. and Yu, G.L., 2009. Rabbit hybridoma. Therapeutic Monoclonal Antibodies: From Bench to Clinic (Zhiqiang An (Editor)) Hoboken.
Ridder R, Schmitz R, Legay F, Gram H. Generation of rabbit monoclonal antibody fragments from a combinatorial phage display library and their production in the yeast Pichia pastoris. Biotechnology 1995; 13: 255–260


.png)