For the second installment in our ABclonal Webinar Series, we had the privilege of inviting Dr. Clarke Gasper, our Business Development Scientist, to share his insights on the production and development of monoclonal antibodies for cancer therapy. If you were unable to attend the live session or would like to review some of Dr. Gasper’s key points, we’ve got you covered with a recap of his lecture below.
Introduction & Overview:
Dr. Gasper’s presentation on monoclonal antibodies in cancer therapy was divided into the following 4 subtopics (click the bullet points below to jump to a specific section):
- A general introduction to antibodies, their structure, and properties
- The various methods for developing monoclonal antibodies
- Monoclonal antibodies for cancer therapeutic use
- Current FDA-approved antibody drugs available in the U.S. market
Section 1: General Introduction to Antibodies
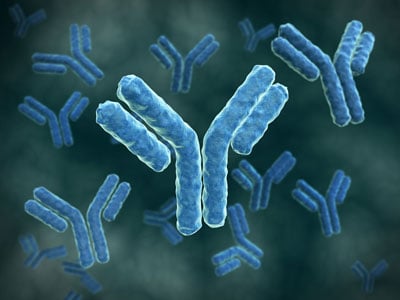
For the first section of the webinar, Dr. Gasper discussed an overview of antibodies, specifically examining their properties and structure. As he outlined, an antibody is a protein that is used by the immune system to identify and neutralize foreign objects, such as bacteria, viruses, and other foreign cells. They recognize specific antigens (as shown in green in Figure 1 below) that bind to antibodies with high-affinity. With respect to its mechanism of action, the antigens usually bind to the variable region on the antibody’s Y-shaped structure, which is composed of two heavy chains (blue in Figure 1) and two light chains (red in Figure 1). Each of these chains is held together by stable, disulfide bonds.
Figure 1. Structure of an IgG antibody
The antibody shown above in Figure 1 falls under the IgG subtype, which make up about 85% of the antibodies found circulating in blood serum. The other isotypes are far less abundant and are only found in specific types of tissue. For this reason, IgG antibodies are the main isotype pursued in therapeutics. Antibodies interact with antigens with non-covalent bonds, which are reversible and serve to determine both the specificity and sensitivity of their interaction.
Section 2: Developing Monoclonal Antibodies
As Dr. Gasper discussed during his webinar, there are four main platforms that can be utilized to develop monoclonal antibodies.
1) Hybridoma Technology
For the mouse (or rabbit hybridoma method, developed later in the 1990s), the animal is first injected with an antigen. The immune system of the animal will begin producing antibodies in response to this antigen. Several rounds of screenings are then performed to verify the antigen specificity of the antibodies. Once the titer is deemed sufficient, the screen that holds the B cells is removed from the animal, and the B cells within the spleen are infused with myeloma cells to create an immortal cell line. At this point you now have a cell whose sole purpose is to produce monoclonal antibodies, and with additional rounds of screenings, a hybridoma cell line that only produces antibodies of interest can be identified. This method was pioneered in the mid-1970s by César Milstein, Georges Köhler, and Niels Kaj Jerne, who ended up sharing the Nobel Prize in Physiology in 1984 for their monoclonal antibody discoveries.
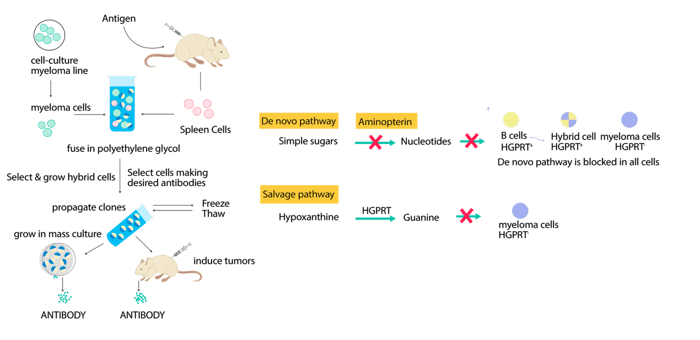
Figure 2. Workflow of the mouse hybridoma method for mAb production.
2) Rabbit B Cell Cloning Technology
Similar to the mouse hybridoma method, the rabbit B cell cloning method begins with immunizing rabbits with antigen. Once determine positive titer screens are determined, B cells are then isolated from the spleen of rabbits; but rather than fusing them and creating hybridoma cell lines, the cells are run through fluorescence-activated cell sorting (FACS) technology that can identify single B cells that are producing antibodies against the antigen of choice. After the B cells are screened usings FACS, the RNA can then be sequenced to obtain the DNA coding sequence for the identified antibody. This sequence can then be cloned into expression vectors, which are used to transfect mammalian cell lines and have them express the antibody of interest.
Rabbit monoclonal antibodies (mAbs) have a few advantages over their mouse-derived counterparts. The immune system of the rabbit is more complicated than that of the mouse, and therefore tends to generate antibodies with higher specificity and affinity. In addition, rabbit mAbs are frequently recommended when experiments are not generating an adequate immune response from a mouse system. A summarized list of comparisons between the two types of monoclonal antibodies can be found below in Figure 3:
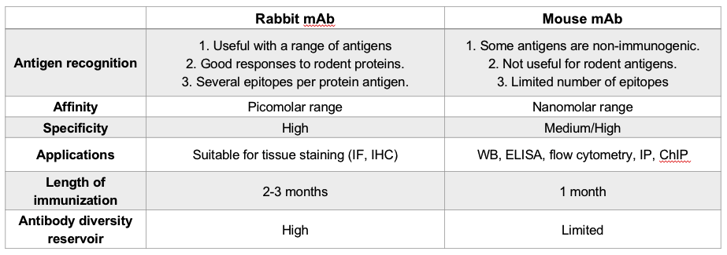 Figure 3. A comparison between mouse and rabbit monoclonal antibodies.
Figure 3. A comparison between mouse and rabbit monoclonal antibodies.
3) Phage Display Library Technology
Unlike the first two platforms mentioned, the phage display technique does not use animals. Rather, it starts with the construction of a large antibody library consisting of IgG heavy and light chains cloned from rabbit, mouse, or human B cells. This large library is placed into M13 bacteriophages, who then display a fragment of the antibody on their surface. The phages can be screened against the antigen of choice, to determine which heavy and light chain pairing is specific to the antigen of interest. After several rounds of artificial screening, a specific heavy and light chain pairing will result that demonstrates high affinity towards the target of interest.
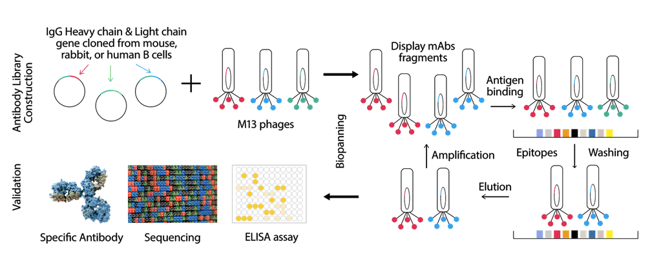 Figure 4. An overview of the phage display technique workflow.
Figure 4. An overview of the phage display technique workflow.
4) Transgenic Mice
This method utilizes transgenic mice whose DNA sequences have been modified so that the antibodies they produce are fully humanized. This technique begins with mouse embryonic stem cells, and uses technology such as CRISPR to insert human genes and edit out/inactivate mouse genes that are associated with heavy and light chains. The two mice, one with human antibody genes and the other with inactivated mouse heavy and light chains, can be bred to produce a XenoMouse that produces fully humanized antibodies. In terms of workflow, the process is similar to that of the hybridoma system, except that the hybridomas created will it result in fully humanized mAbs as shown in Figure 4 below:
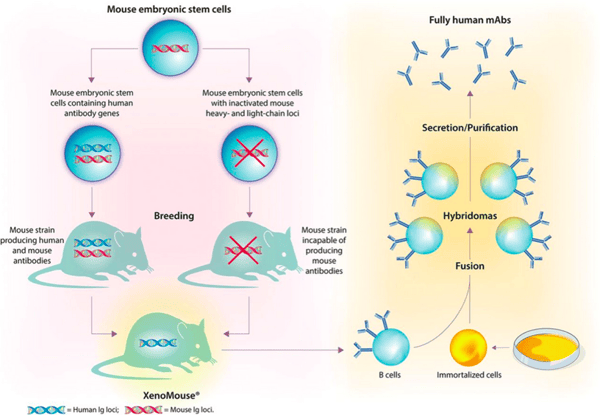
Figure 5. Workflow for the transgenic mouse method to produce fully humanized monoclonal antibodies.
Section 3: Monoclonal Antibodies for Cancer Therapeutic Use
There are three main classes of monoclonal antibodies for cancer therapeutic use: naked, conjugated, and bispecific.
1) Naked Antibodies
Naked antibodies are mAbs without drugs or radioactive material attached. They are used to mark cells for degradation in the immune system, and to attach to receptors and block binding of growth factors and cytokines. Two popular naked antibody-based cancer drugs on the marketplace are Herceptin (trastuzumab), targeting HER-2 for breast cancer treatment, and Rituxan (rituximab), which targets B cells in non-Hodgkin lymphoma specifically binding to the protein CD20. With respect to their market share, seven of the top twenty selling drugs in 2017 were antibody-based, with over 810 additional therapeutics in the clinical trials stage.
2) Conjugated Antibodies
Conjugated antibodies are mAbs conjugated with chemotherapeutic agents. These antibodies have a linker that provides them with a payload that can be used as a pharmalogic, radioactive, or cytotoxic cancer therapeutic. Figure 5 below depicts the mechanism of action for antibody-drug conjugates:
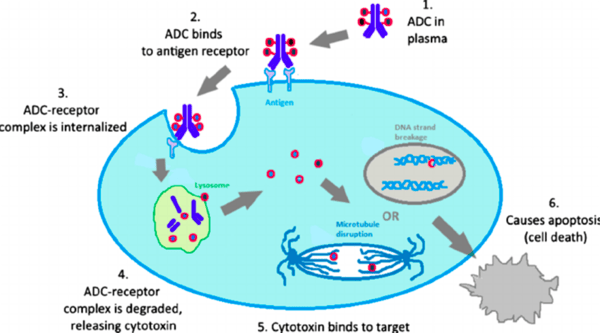
Figure 6. Mechanism of action for antibody-drug conjugates.
The antibody-drug conjugate (ADC) first binds to a receptor on surface of the tumor, and is then internalized into the tumor cell and degraded. Once the ADC that is carrying the therapeutic is degraded, the therapeutic can then act specifically to prevent the tumor cells from further division. The main goal of ADC therapeutics are to provide targeted delivery of therapeutic chemicals. Challenges to this method are the possibility of off-target effects, caused by an unstable linker within the ADC after administration of the agent, as well as heterogeneity of the target due to varying levels of conjugation.
3) Bispecific Antibodies
Bispecific antibodies are hybrid antibodies, with epitopes engineered to react to a variety of different cells. They are created by joining together two halves of two different antibodies with specific epitopes, to customize the therapeutic to specific targets of interest. Bispecific antibodies are further subdivided into two categories: IgG-like, and non-IgG-like. IgG-like antibodies have customized epitopes to coordinate how the immune system recognizes tumor cells. For example, a triomab has three receptors for antigens on each end of its structure. The Fc receptor recruits accessory cells from the immune system (i.e. NK cells, macrophages), which then stimulates the recruitment of T cells to the region that bind to a separate epitope. Finally, the third epitope is exposed and binds to the tumor cell. Figure 6 below shows a detailed diagram of the triomab's binding mechanism:
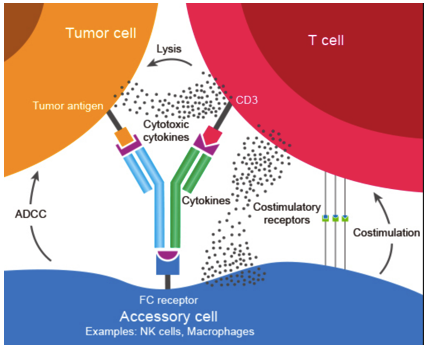 Figure 7. Structure and binding mechanism of a triomab, an example of an IgG-like bispecific antibody.
Figure 7. Structure and binding mechanism of a triomab, an example of an IgG-like bispecific antibody.
Non-IgG-like antibodies can take a variety of different forms, such as nanobodies that have a single heavy chain and are able to be expressed in more linear systems such as E. coli. One advantage to non-IgG-like bispecific antibodies is their small size, which allows them the ability to penetrate deeper into tumors. However, they are less stable than their IgG-like counterparts, and therefore have a shorter half-life.
Section 4: Popular FDA-approved Antibody-Based Drugs in the US Market
The table lists some of the most well-known antibody drugs currently approved by the Food and Drug Administration (FDA), as well as their respective therapeutic targets.
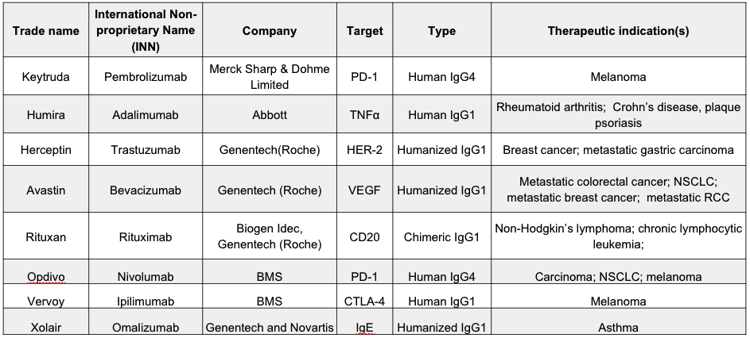
Figure 8. Well-known antibody drugs currently approved by the FDA.