In direct support of the fight against the novel coronavirus and the world's effort to understand more about the mechanisms of the COVID-19 disease, we're proud to announce the arrival of our SARS-CoV-2 Neutralizing Antibody.
An Introduction to SARS-CoV-2 Neutralizing Antibodies
Our SARS-Cov-2 neutralizing antibodies are isolated from patients infected with the novel coronavirus, and are recombinantly produced using human embryonic kidney 293 (HEK293) cells. The spike protein on the novel coronavirus is a large, transmembrane protein that contains two subunits, known as S1 and S2 respectively. On the S1 portion of the spike protein is a receptor binding domain (RBD) that recognizes the human cell surface receptor ACE2. The neutralizing antibodies recognize the SARS-CoV-2 spike protein RBD, and thus inhibit the binding between the RBD and ACE2, the point of entry receptor located on the human cell. Figure 1 below illustrates the neutralizing antibody mechanism:
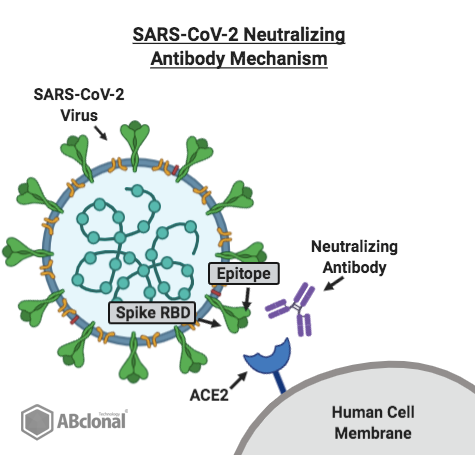 Figure 1. Mechanism of the SARS-Cov-2 neutralizing antibody (purple), blocking viral entry.
Figure 1. Mechanism of the SARS-Cov-2 neutralizing antibody (purple), blocking viral entry.
As illustrated in the diagram above, the neutralizing antibody competes with the ACE2 receptor on the human cell for binding with the receptor binding domain (RBD) on the novel coronavirus spike protein. The epitope on the virus' spike protein functions as an epitope for the neutralizing antibody, as well as a binding site for human ACE2. The competition between the two effectively "neutralizes" the virus and prevents its entry into the human cell through ACE2.
The ABclonal Advantage
Our particular SARS-CoV-2 Neutralizing Antibody features a half maximal inhibitory concentration (IC50) of only 0.496 µg/mL, one of the lowest currently available in the industry. The IC50 is a measure of the effectiveness of a substance in inhibiting a specific biological or chemical function. Its value is the concentration of a drug that is required for 50% inhibition in vitro. In this case, the biological function is the binding of the SARS-CoV-2 spike protein to the ACE2 receptor, and as measured it would take a concentration of only 0.496 µg/mL for our neutralizing antibody to induce 50% inhibition. A bioactivity comparison between ABclonal and two industry leading competitors using the IC50 metric is shown in Table 1 below:
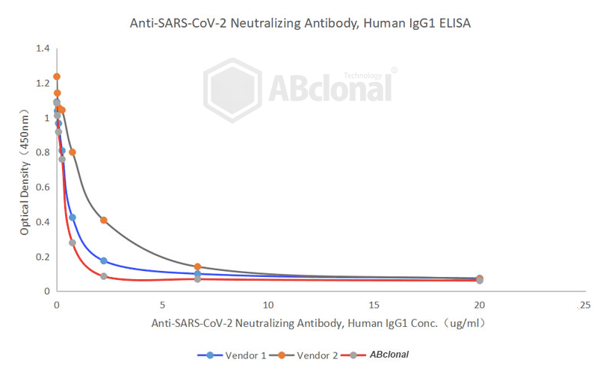 Table 1. Bioactivity comparison shows an IC50 of 0.469 µg/mL for our neutralizing antibody, lower than two leading competitors.
Table 1. Bioactivity comparison shows an IC50 of 0.469 µg/mL for our neutralizing antibody, lower than two leading competitors.
As previously mentioned, our SARS-CoV-2 neutralizing antibodies are recombinantly produced from human cells (HEK293), which ensures the production of reliable antibodies that result in post-translational modifications most consistent with those seen endogenously on human proteins. The spike (S) glycoprotein on the SARS-CoV-2 virus is a key target of neutralizing antibodies upon infection, and the underlying mechanism behind this process is a vital area of COVID-19 research that has yet to be fully understood. As states continue to reopen across the country, neutralizing antibodies against the SARS-CoV-2 virus are therefore all the more important to investigate given their association with potential protective immunity.
Learn more about our new SARS-CoV-2 Neutralizing Antibody here. If you have questions regarding our neutralizing antibodies, or any of our other research reagents and antibody products, reach out to our expert support team using the button below.