CD antigens have played a significant role in both diagnosis and treatment for several diseases ranging from autoimmune diseases to cancer. CD antigens are often used as drug targets in drug discovery and as biomarkers in diagnosis because they are both highly specific and are located at the surface of the cells to target different to identify and investigate cell surface molecules.
What are CD antigens?
“Cluster of Differentiation”, abbreviated as CD is a protocol that helps target immunophenotyping cells. CD antigens, also known as CD markers, are specific group of molecules found on the surface of cell especially on immune cells (T-cell, B-cell, and NK cells etc.) that help them differentiate from each other. They are labeled by numbers and there are over 300 CD markers discovered. The latest CD marker discovered is CD371. CD markers have many functions including cell adhesion, immune response, inflammation response etc. There are several CD targets that are popular in drug discovery such as CD19, CD20 and CD38, to name a few.
CD Marker as a Drug Target
Double stranded break repair:
Regarding CD marker as drug targets, at times, monoclonal antibodies (mAb) are designed to attack the specific CD antigen of interest. There are many FDA-approved monoclonal antibodies drugs in the market, that target various types of antigens including CD markers, treating from autoimmune diseases to cancer and currently COVID-19.
CD markers for targeted cancer treatment
As mentioned above, CD markers are widely used as drug targets for several diseases including cancer. For example, CD38 is used as a target in treating multiple myeloma (MM). Multiple myeloma a form of cancer in plasma cell (a type of white blood cell that fight infections in our body). In multiple myeloma, the cancerous plasma cells accumulate in the bone marrow, crowding out healthy blood cells and causing complications. CD38 is used due to its role in cell adhesion and the over expression of CD38 on MM cells, and this results in antibody treatments for MM. Although multiple myeloma is a relative uncommon cancer, in the United States, about 0.76% of the population is diagnosed with MM and it is the 14th leading cause of cancer deaths.
While there is no cure for this cancer, there are treatments and drugs to help manage this cancer. One of these drugs being Daratumumab (Drazalex), an anti-cancer antibody drug that directly targets CD38 as shown in the figure below; and recently, Isatuximab (Sarclisa), another monoclonal antibody drug targeting CD38 was approved to treat MM.
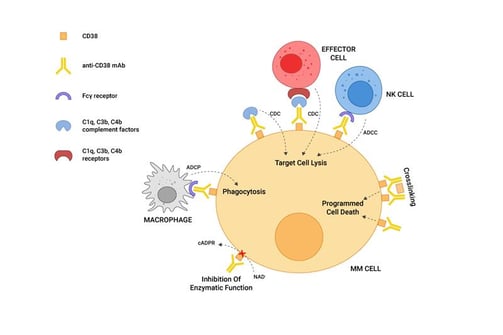
Figure 1: A schematic representation of the mechanism(s) of action of CD38-targeting mAbs on MM cells. Source: Morandi, F., Horenstein, A. L., Costa, F., Giuliani, N., Pistoia, V., & Malavasi, F. (1AD, January 1). Cd38: A target for immunotherapeutic approaches in multiple myeloma. Frontiers. https://www.frontiersin.org/articles/10.3389/fimmu.2018.02722/full
Several approved mAb drugs targeting CD antigens
|
Drug name |
Target |
Type of treatment |
Disease |
|
Pembrolizumab (Keytruda) |
PD-1/ |
Immunotherapy |
melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, and stomach cancer. |
|
Rituximab (Rituxan) |
non-Hodgkin's lymphoma, chronic lymphocytic leukemia, relapsed diffuse large B cell lymphoma (DLBCL). |
||
|
Daratumumab (Drazalex) |
Immunotherapy (anti-cancer drug) |
Multiple Myeloma |
|
|
Isatuximab (Sarclisa) |
Chemotherapy |
Multiple Myeloma |
|
|
trastuzumab (Herceptin) |
Targeted therapy (part of chemotherapy) |
Breast cancer, stomach cancer |
CD marker and CAR-T Therapy
Targeted therapy like trastuzumab (Herceptin), using drugs that target the molecular pathway changes of specific cancer cells has been the standard treatment for cancer. Over the past few years, immunotherapy like Chimeric Antigen Receptor (CAR)-T cell therapy has been emerging in cancer treatment. In CAR-T cell therapy, T-cells are modified to produce a specific receptor on their cell surface and once the T-cells are re-infused into the blood, they then targets specific antigens, killing tumor cells. Take CD19 as an example, CARs targeting the B cell antigen CD19 have been studied extensively to treat B cell malignancies. CD19 makes an attractive target for CAR-T cell therapy because of its expression on most B cell malignancies.
Kymriah, a CD19-directed CAR therapy was the first CAR T-cell therapy approved for the treatment of relapsed/refractory B cell acute lymphoblastic leukemia (B-ALL) and Yescarta, another CD19-directed CAR therapy was approved to treat relapsed diffuse large B cell lymphoma (DLBCL). The structure and mechanism of Kymriah can be seen in the figure below.
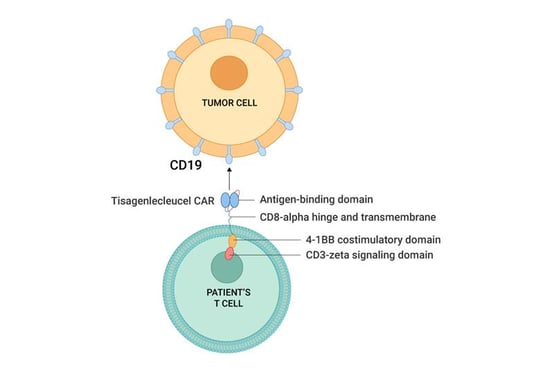 Figure 2: Mechanism of Tisagenlecleucel CAR, Kymriah (pink) directly targets CD19 on the tumor cell.
Figure 2: Mechanism of Tisagenlecleucel CAR, Kymriah (pink) directly targets CD19 on the tumor cell.
Source: 1Department of Pediatric Hematology and Oncology. (n.d.). Chimeric antigen receptor-t cell therapy: Practical... : Hemasphere. LWW. https://journals.lww.com/hemasphere/Fulltext/2018/02000/Chimeric_Antigen_Receptor_T_Cell_Therapy_.1.aspx.
Several approved CAR-T cell therapies targeting CD19 antigens
|
Drug name |
Target |
Type of treatment |
Disease |
|
Tisagenlecleucel (Kymriah) |
relapsed/refractory B cell acute lymphoblastic leukemia (B-ALL) |
||
|
Axicabtagene ciloleucel (Yescarta) |
relapsed diffuse large B cell lymphoma (DLBCL). |
||
|
Brexucabtagene autoleucel (Tecartus) |
mantle cell lymphoma |
||
|
lisocabtagene maraleucel (Breyanzi) |
relapsed or refractory large B-cell lymphoma (R/R LBCL) |
What ABclonal Can Do
ABclonal offers a variety of catalog CD proteins, antibodies, custom antibody services and ELISA kits covering many popular research areas. We have currently over 900 CD products with multiple applications that are currently in our inventory. Currently our featured CD product focuses on disease like Multiple Myeloma, COVID-19 and Chronic Myeloid Leukemia. ABclonal is also committed to continue development of additional CD products to meet the growing market demand.
References
Car t cells: Engineering immune cells to treat cancer. National Cancer Institute. (n.d.). https://www.cancer.gov/about-cancer/treatment/research/car-t-cells.
Fleischer, L. C., Spencer, H. T., & Raikar, S. S. (2019, December 29). Targeting t cell malignancies using car-based immunotherapy: Challenges and potential solutions. Journal of Hematology & Oncology. https://jhoonline.biomedcentral.com/articles/10.1186/s13045-019-0801-y.
Greenwood, M. (2021, January 15). What are cd markers? News. https://www.news-medical.net/life-sciences/What-are-CD-Markers.aspx.
Key statistics for multiple myeloma. American Cancer Society. (n.d.). https://www.cancer.org/cancer/multiple-myeloma/about/key-statistics.html.
Mayo Foundation for Medical Education and Research. (2021, June 16). Multiple myeloma. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/multiple-myeloma/symptoms-causes/syc-20353378.
Mullard, A. (2021, May 5). FDA approves 100th monoclonal ANTIBODY PRODUCT. Nature News. https://www.nature.com/articles/d41573-021-00079-7.
Nozari, A., & Berezovski, M. V. (2017). Aptamers for CD Antigens: From Cell Profiling to Activity Modulation. Molecular therapy. Nucleic acids, 6, 29–44. https://doi.org/10.1016/j.omtn.2016.12.002
Scheuermann, R. H., & Racila, E. (1995). CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leukemia & lymphoma, 18(5-6), 385–397. https://doi.org/10.3109/10428199509059636
van de Donk, N., & Usmani, S. Z. (2018). CD38 Antibodies in Multiple Myeloma: Mechanisms of Action and Modes of Resistance. Frontiers in immunology, 9, 2134. https://doi.org/10.3389/fimmu.2018.02134