My wife and I used to watch House, M.D. starring Hugh Laurie, in which he was a cranky doctor who happened to be a Holmesian genius in diagnosing rare or mysterious diseases. We are fortunate to have doctors who have much better bedside manner, but as an entertainment option, House was a lot of fun. One of the running gags for fans of the show is that the mystery disease of the week is never lupus, except for the one and only time that it was. My fond memories of this show got me to thinking about how difficult it is to diagnose lupus, and about other autoimmune diseases that still remain mysterious and challenging to treat. I decided to find out how modern medicine is approaching this continuing health issue.
My wife and I used to watch House, M.D. starring Hugh Laurie, in which he was a cranky doctor who happened to be a Holmesian genius in diagnosing rare or mysterious diseases. We are fortunate to have doctors who have much better bedside manner, but as an entertainment option, House was a lot of fun. One of the running gags for fans of the show is that the mystery disease of the week is never lupus, except for the one and only time that it was. My fond memories of this show got me to thinking about how difficult it is to diagnose lupus, and about other autoimmune diseases that still remain mysterious and challenging to treat. I decided to find out how modern medicine is approaching this continuing health issue.
Targeted Monoclonal Therapies and Biomarkers
Early attempts to treat autoimmune disorders such as lupus, multiple sclerosis, and others included broad immunosuppressants, but because of the non-specific action of these medications, patients often experience unpleasant side effects. 1 Precision therapies eventually came about, including small molecule inhibitors against candidate pro-inflammatory cytokines and their receptors, including Janus kinase (i.e. JAK1). 1
As you may have seen in those medication commercials (where they spend about 15 seconds saying what it’s for and then the other 45 seconds listing all the fun side effects), many drugs nowadays are based on humanized monoclonal antibodies against a specific target. 1 Similar strategies are employed for autoimmune diseases, including systemic lupus erythematosus, for which one treatment is the monoclonal antibody belimumab. 2 Beliumumab is designed to target the B lymphocyte stimulator (BLyS), also known as the B cell-activating factor (BAFF), which is upregulated in lupus patients and is known to drive disease progression. 2
The death receptor CD95 (Fas) and its ligand CD95L (Fas ligand, or FasL) are involved in the regulation of the immune system through the mechanism of cytotoxic T lymphocytes killing target cells, as well as in eliminating activated T cells through apoptotic cell death. 3 Aberrant expression or mutation of CD95 and CD95L could result in abnormal cell death or in the persistence of autoreactive T cells, making these proteins attractive targets in studying autoimmunity. 3 In multiple sclerosis, CD95L is used as a prognostic biomarker to detect relapses, while targeting TNFR1, TRAIL receptor, and CD95 in concert appeared to have some effect in reducing inflammation in lupus mouse models. 3
Solid Gold: Using Nanotechnology to Manage Autoimmune Diseases
 An intriguing new strategy against autoimmune disorders is leveraging the unique properties of gold and other metal nanoparticles. There are reports that gold nanoparticles (GNP) have anti-inflammatory effects and might be useful treatments against type I diabetes, rheumatoid arthritis, and inflammatory bowel diseases. 4 In addition to being flexible and malleable, the GNPs can carry therapeutics to their targets as well as protect them from degradation. GNPs can be engulfed by antigen presenting cells through phagocytosis, and appear to have few, if any, cytotoxic effects. 4 The GNPs may inhibit the production of pro-inflammatory cytokines as well as reduce the release of reactive oxygen species. 4
An intriguing new strategy against autoimmune disorders is leveraging the unique properties of gold and other metal nanoparticles. There are reports that gold nanoparticles (GNP) have anti-inflammatory effects and might be useful treatments against type I diabetes, rheumatoid arthritis, and inflammatory bowel diseases. 4 In addition to being flexible and malleable, the GNPs can carry therapeutics to their targets as well as protect them from degradation. GNPs can be engulfed by antigen presenting cells through phagocytosis, and appear to have few, if any, cytotoxic effects. 4 The GNPs may inhibit the production of pro-inflammatory cytokines as well as reduce the release of reactive oxygen species. 4
Working in Tandem: Fusion Proteins
Another clever approach is in setting up a chimeric system, or a fusion protein that is made of parts of two or more unique proteins. In the context of CD95L, binding CD95L to a moiety such as streptavidin could induce immunosuppression. 3 In another application, the extracellular domains of both CD95L and PD-L1 are joined to inhibit activated T cells, which are known to express the PD-1 receptor. 3 Because many immune cells communicate via bidirectional receptor-ligand signaling, it will be interesting to see if other fusion proteins could be created to modulate the immune response and alleviate symptoms of autoimmunity.
Regulators, Mount Up: Designing Novel Regulatory T Cell-Based Therapies
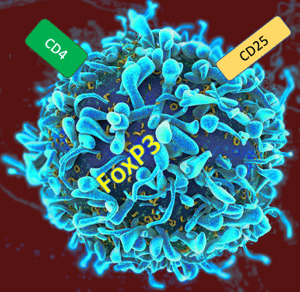 We know that there is a huge role of regulatory T cells, or Tregs, in tumor immunology, and it only makes sense that they would be crucial to understanding autoimmunity as well. Autoimmune disorders are the result of disruptions in immune tolerance, which also suggest that Treg function has gone awry. Similar to chimeric antigen receptor (CAR) T cells in various therapies, including against cancer, there are studies underway to determine whether Treg cell specificity and function can be modulated to induce a balanced immune suppression to curb autoimmune symptoms. 1, 5 As a more nuanced strategy, it makes sense to reprogram a Treg cell to seek out and stop autoreactive cells rather than shutting down the entire immune system with immunosuppressive drugs. Ultimately, as scientists continue to learn more about immune regulation and how to properly tweak the regulatory functions of these cells, the aim is to find a way to restore immune balance while maintaining a functional immune system against actual foreign antigens and disease. As a bonus, a precisely reprogrammed Treg cell could be used as a “naturalized” medication against transplant rejection in lieu of an immunosuppressant.
We know that there is a huge role of regulatory T cells, or Tregs, in tumor immunology, and it only makes sense that they would be crucial to understanding autoimmunity as well. Autoimmune disorders are the result of disruptions in immune tolerance, which also suggest that Treg function has gone awry. Similar to chimeric antigen receptor (CAR) T cells in various therapies, including against cancer, there are studies underway to determine whether Treg cell specificity and function can be modulated to induce a balanced immune suppression to curb autoimmune symptoms. 1, 5 As a more nuanced strategy, it makes sense to reprogram a Treg cell to seek out and stop autoreactive cells rather than shutting down the entire immune system with immunosuppressive drugs. Ultimately, as scientists continue to learn more about immune regulation and how to properly tweak the regulatory functions of these cells, the aim is to find a way to restore immune balance while maintaining a functional immune system against actual foreign antigens and disease. As a bonus, a precisely reprogrammed Treg cell could be used as a “naturalized” medication against transplant rejection in lieu of an immunosuppressant.
"Inverse" Vaccines
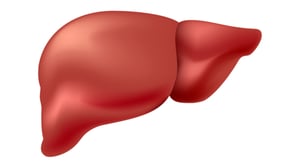 In the late summer of 2023, researchers from the University of Chicago introduced the concept of an "inverse" vaccine that could alleviate the course of autoimmune diseases in a lab setting. Unlike other immunosuppression-based treatments, however, this new strategy does not also shut down the rest of the immune system. As of this writing, human trials are being initiated, but the promise of a more targeted autoimmune therapy is always exciting. The report, published in Nature Biomedical Engineering, 6 showed that a synthetic glycosylated antigen could be used in a mouse EAE (multiple sclerosis) model to establish tolerance against that specific antigen and reduces the inflammatory responses associated with adverse symptoms of that disease. The idea behind this type of "inverse vaccine" is to remove a response against a specific triggering antigen, rather than against all other antigens, so that the therapy helps prevent those adverse symptoms while keeping the rest of the immune system primed to act against potential infections and tumors. By tagging their experimental antigens with a sugar called N-acetylgalactosamine (pGal), this directs the antigen to the liver where tolerance is established and those adverse symptoms are reversed, allowing the mouse nervous system to recover and function normally again. Researchers still need to delineate which antigens are the triggering self-antigens to make this strategy effective for more diseases, and will continue to determine how the liver helps establish immune tolerance, but the hope is that this approach can be translated broadly to other autoimmune diseases like lupus. You can learn more from the Science overview of this potentially transformative research.
In the late summer of 2023, researchers from the University of Chicago introduced the concept of an "inverse" vaccine that could alleviate the course of autoimmune diseases in a lab setting. Unlike other immunosuppression-based treatments, however, this new strategy does not also shut down the rest of the immune system. As of this writing, human trials are being initiated, but the promise of a more targeted autoimmune therapy is always exciting. The report, published in Nature Biomedical Engineering, 6 showed that a synthetic glycosylated antigen could be used in a mouse EAE (multiple sclerosis) model to establish tolerance against that specific antigen and reduces the inflammatory responses associated with adverse symptoms of that disease. The idea behind this type of "inverse vaccine" is to remove a response against a specific triggering antigen, rather than against all other antigens, so that the therapy helps prevent those adverse symptoms while keeping the rest of the immune system primed to act against potential infections and tumors. By tagging their experimental antigens with a sugar called N-acetylgalactosamine (pGal), this directs the antigen to the liver where tolerance is established and those adverse symptoms are reversed, allowing the mouse nervous system to recover and function normally again. Researchers still need to delineate which antigens are the triggering self-antigens to make this strategy effective for more diseases, and will continue to determine how the liver helps establish immune tolerance, but the hope is that this approach can be translated broadly to other autoimmune diseases like lupus. You can learn more from the Science overview of this potentially transformative research.
The Promise of a Better Tomorrow
As genetic modification technology improves and the regulatory mechanisms of the immune system are elucidated, I am encouraged that we will see much more effective treatments within our lifetimes against the spectrum of autoimmune diseases that still plague the human population. Fine-tuning a targeted therapy is a monumental task for any disease, including when it actually is lupus. With every new piece of knowledge, coupled with a heaping spoonful of human ingenuity, we are ever closer to our collective goal of improving global health and our quality of life.
References
- Fugger L, Jensen LT & Rossjohn J (2020) “Challenges, Progress, and Prospects of Developing Therapies to Treat Autoimmune Diseases.” Cell 181(1):63-80.
- Dubey et al. (2011) “Belimumab: First targeted biological treatment for systemic lupus erythematosus.” J Pharmacol Pharmacother 2(4):317-319.
- Risso V, Lafont E & Le Gallo M (2022) “Therapeutic approaches targeting CD95L/CD95 signaling in cancer and autoimmune diseases.” Cell Death Dis 13(3):248 (Epub).
- Koushki et al. (2021) “Gold Nanoparticles: Multifaceted Roles in the Management of Autoimmune Diseases.” Biomolecules 11(9):1289 (Epub).
- Ferreira et al. (2019) “Next-Generation regulatory T cell therapy.” Nat Rev Drug Discov 18(10):749-769.
- Tremain et al. (2023) "Synthetically glycosylated antigens for the antigen-specific suppression of established immune responses." Nature Biomedical Engineering 7:1142-1155.