The 2021 Nobel Prize in Physiology of Medicine was awarded jointly to David Julius, of the University of California at San Francisco, and Ardem Patapoutian, a neuroscience researcher at the Scripps Research Institute in La Jolla, California. Working independently, Julius and Patapoutian discovered the key receptors (TRPV1, TRPM8, Piezo1, and Piezo2) in our bodies that sense heat, cold, and touch. Their work not only helps us to understand how we perceive and adapt to the surrounding world, but also paves the way for drug discoveries that target a wide range of diseases, including chronic pain, respiratory disease, and cancer.
The Way You Make Me Feel: Pondering the Mechanisms of Sensation
Humans have been curious about the sources of sensations, such as smell, sight, pain, and touch, since the earliest days of recorded history as the ancient Babylonians recorded references to pain on clay tablets. While studies elucidating the mechanisms of sight and smell were performed relatively early in the 20th Century, human understanding of the perceptions of pain and touch remained a mystery. In ancient times, pain was considered a spiritual, mystical experience. The 17th Century French philosopher, René Descartes, was among the first connecting the sensing of pain due to excessive heat to the brain. Descartes described pain as a perception that resulted from a disturbance in the sensory organs (for example, the skin) that was then passed along nerve fibers that ultimately reached the brain. This model later led to our modern understanding of the nervous system.

FIGURE 1: Rodin's "The Thinker," an appropriate metaphor for human contemplation.
In the years that followed, many scientists have strived to unravel the detailed mechanism that converts temperature and mechanical stimuli into nerve impulses. This includes the remarkable achievements by David Julius and Ardem Patapoutian in discovering key sensory receptors that have enhanced our knowledge of human perception.
Hot and Bothered: Translating Heat Into Nervous Signals
A compound called capsaicin elicits the burning sensation when we bite into a chili pepper, whether intentionally or unknowingly, as it activates sensory neurons to transmit the noxious stimuli to the brain. After guzzling down several cups of water to soothe the pain, one might wonder how capsaicin is recognized by the cells on our tongue and in our cheeks. In 1997, a team led by David Julius discovered the protein receptor that binds capsaicin by screening a cDNA library of approximately 16,000 clones that were transfected into HEK293 cells. 1 While meticulously searching for cells that responded to capsaicin with calcium influx, they were able to find a single clone encoding the transient receptor potential cation channel subfamily V member 1, or TRPV1, which has the ability to sense elevated temperatures.
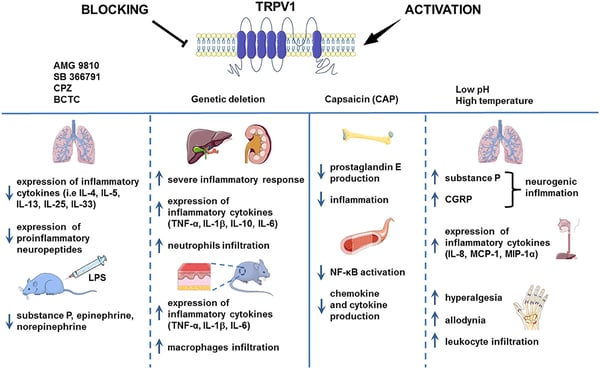
FIGURE 2: TRPV1 is often implicated in the inflammatory response. Source: Bujak et al. (2019) 2
TRPV1 is a ligand-activated non-selective cation channel mainly expressed in nociceptive neurons, which are preferentially sensitive to noxious and painful stimuli. 3 The receptor contains six transmembrane helices and its biological functions, including inflammatory responses, are primarily mediated from sodium and calcium signaling pathways. (Figure 2) Upon activation by capsaicin or heat, TRPV1 undergoes a transition from closed- to open-gate, allowing sodium and calcium ions to flow into the neuron, thereby depolarizing the cell. 4 This leads to the firing of action potentials and the sensation of spiciness and pain. In addition to detecting spicy foods, TRPV1 is also responsible for the detection and transduction of stimuli such as temperatures greater than 43 °C, arachidonic acid metabolites, jellyfish and spider toxins, and vanilloids. 5, 6 Learn more about our TRPV1 antibody.
Cold as Ice: The Receptor That Senses Cold and Menthol
The discovery of TRPV1 led to studies to unveil other temperature-sensing receptors. Identified independently by both Julius and Ardem Patapoutian’s group in 2002, the transient receptor potential cation channel subfamily M member 8, or TRPM8, can sense cold and menthol. 7, 8 After chewing certain types of gum or popping an Altoid, the cooling sensation of menthol in one’s mouth is transduced by TRPM8 through a calcium-dependent signaling pathway regulated by phosphatidylinositols and the activation of phospholipase C. 9 TRPM8 is responsible for sensing temperatures in the range of 8 °C to 28 °C in addition to cooling compounds such as menthol. Interestingly, coughing and airway constriction in response to cold have been shown to result from TRPM8 activation. 10 TRPM8 has also been associated with melanoma, breast cancer, pancreatic cancer, and prostate cancer, making this receptor a promising biomarker for the diagnosis and treatment of those maladies. 10
With both discoveries of TRPV1 and TRPM8 catalyzing further research, new studies in thermosensation have revealed novel receptors including TRPC5, TRPM3, and TRPA1. In concert with each other, these receptor channels contribute to the delicate sensory machinery in the skin to detect a wide range of temperatures from the tolerable to the extremely uncomfortable. 11 Learn more about our TRPM8 antibody.
Under Pressure: Deciphering the Biology of Touch
The last mysterious perception was clarified through a breakthrough discovery by Patapoutian’s group in 2010 in identifying two receptors that detect pressure and mechanical force. Named Piezo1 and Piezo2, these receptors are transmembrane proteins similar to TRPV1 and TRPM8. (Figure 3) The Piezo receptors are ion channels which are essential for the conversion of mechanical stimuli into biological signals, a process called mechanotransduction. 12
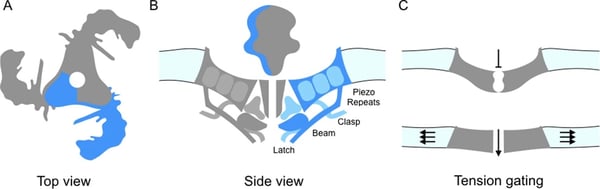
FIGURE 3: The Piezo1 ion channel. A) The trimer is oriented in the cell membrane to produce a central pore. B) Side view of Piezo1 in the cell membrane. C) Piezo1 pore will open as a result of membrane tension. Source: Chesler & Szczot (2018) 16
Over the next decade, extensive studies focused on Piezo1 and Piezo2 have revealed many new insights. For example, Piezo 2 was shown to be a major regulator of the perception of body and limb position, or proprioception. 13 Aberrant expression of Piezo1 can cause uncoordinated body movements. Piezo1 has also been associated with various biological processes including maintaining red blood cell homeostasis and regulating vascular development. 14, 15 Learn more about our Piezo1 antibody.
A New Sensation: Insights From the Study of Sensory Receptors
The significance of the discovery of these neuroreceptors is not understated, as their clinical applications are immediately apparent in the development of targeted therapies. Small antagonistic molecules targeting TRPV1 and TRPM8 are being pursued as analgesic agents against chronic pain. 17 A recent study has also correlated Piezo channels to migraine pain. 18 Furthermore, both TRPV1 and TRPM8 have been linked to the onset of respiratory diseases including asthma, chronic coughing, and lung disease, making them potential targets for treatments. 17 The discovery that TRPV1 and TRPM8 expression is altered in different types of cancers also suggests their utility in developing antitumor therapies. 17
How ABclonal Can Help
ABclonal Technology is an original antibody and biological reagents manufacturer that offers a wide array of antibody reagents, custom antibody services, and ELISA kits covering many neurological markers, including the TRPV, TRPM, and Piezo receptors. We aim to support your further research into sensation and the association of these molecules in various disease states. Please click to view our neuroscience research products, and our sensory receptor reagents are listed below:
|
Target |
Cat. No. |
Product |
|
TRPV1 |
TRPV1 Rabbit pAb |
|
|
Mouse TRPV1 ELISA Kit |
||
|
TRPV2 |
TRPV2 Rabbit pAb |
|
|
TRPV5 |
TRPV5 Rabbit pAb |
|
|
TRPV6 |
TRPV6 Rabbit pAb |
|
|
TRPM2 |
TRPM2 Rabbit pAb |
|
|
TRPM4 |
TRPM4 Rabbit pAb |
|
|
TRPM5 |
TRPM5 Rabbit pAb |
|
|
TRPM7 |
TRPM7 Rabbit pAb |
|
|
TRPM8 |
TRPM8 Rabbit mAb |
|
|
TRPM8 Rabbit pAb |
||
|
TRPM8 Rabbit pAb |
||
|
PIEZO1 |
PIEZO1 Rabbit pAb |
|
|
PIEZO2 |
PIEZO2 Rabbit pAb |
REFERENCES
- Caterina et al. (1997) “The capsaicin receptor: a heat-activated ion channel in the pain pathway.” Nature 389:816-824.
- Bujak et al. (2019) “Inflammation, Cancer and Immunity—Implication of TRPV1 Channel.” Front. Oncol. 9:01087 (Epub).
- Dubin and Patapoutian (2010) “Nociceptors: the sensors of the pain pathway.” J Clin Invest 120(11):3760-3772.
- Yang and Zhang (2017) “Understand spiciness: mechanism of TRPV1 channel activation by capsaicin.” Protein Cell 8:169-177.
- Elokely et al. (2016) “Understanding TRPV1 activation by ligands: Insights from the binding modes of capsaicin and resiniferatoxin.” PNAS 113:E137-E145.
- Tominaga et al. (1998) “The cloned capsaicin receptor integrates multiple pain-producing stimuli.” Neuron 21:531-543.
- McKemy et al. (2002) “Identification of a cold receptor reveals a general role for TRP channels in thermosensation.” Nature 416:52-58.
- Peier et al. “A TRP channel that senses cold stimuli and menthol.” Cell 108:705-715.
- Yudin and Rohacs (2012) “Regulation of TRPM8 channel activity.” Mol Cell Endocrinol 353:68-74.
- Liu et al. “TRPM8 channels: A review of distribution and clinical role.” European Journal of Pharmacology 882:173312 (Epub).
- Wang & Siemens (2015) “TRP ion channels in thermosensation, thermoregulation and metabolism.” Temperature (Austin) 2:178-187.
- Coste et al. (2010) “Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels.” Science 330:55-60.
- Woo et al. (2015) “Piezo2 is the principal mechanotransduction channel for proprioception.” Nat Neurosci 18:1756-1762.
- Ranade et al. (2014) “Piezo1, a mechanically activated ion channel, is required for vascular development in mice.” PNAS 111:10347-10352.
- Cahalan et al. (2015) “Piezo1 links mechanical forces to red blood cell volume.” eLife 4:e07370.
- Chesler and Szczot (2018) “Piezo Ion Channels: Portraits of a pressure sensor.” eLife 2018;7:e34396 (Epub).
- Koivisto et al. (2021) “Advances in TRP channel drug discovery: from target validation to clinical studies.” Nat Rev Drug Discov 21:41-59.
- Della Pietra et al. (2020) “The Emerging Role of Mechanosensitive Piezo Channels in Migraine Pain.” Int J Mol Sci 21:E696.