With a background in both immunology and cancer biology, I’ve always had a fascination with the interplay between the body’s immune system and any tumors that might pop up. Originally, it made sense that the immune system would actively seek out and destroy cancerous cells, but the emerging consensus is that the interactions between cancers and host immunity is far more complex. In addition to growing new blood vessels and reprogramming metabolic processes, there appears to be some imbalance between avoiding immune cells while also promoting tumor-infiltrating inflammatory cells to promote its growth. 1 (Figure 1) Trying to dissect this apparent contradictory relationship between tumors and host immunity remains a hot topic.

Figure 1: Tumors have an interesting decision to make in determining which parts of the immune system they want to recruit and avoid.
The Role of Regulatory T Cells in Immunity and Cancer
If immune cells such as natural killer cells are primed to hunt down and destroy cancers, then logically any surviving cancer cells should secrete immunosuppressive molecules or hide their abnormal receptors from the immune patrols. 1 Soon after the discovery of immune “brakes” such as CTLA-4 and PD-1—discoveries that were awarded the 2018 Nobel Prize in Physiology or Medicine—there was a new wave of therapies designed around removing the suppression of the immune system in treating cancers. One of the main targets of these therapies is the regulatory T cell, also known as Tregs, which are capable of tempering immune responses and might be coopted by the tumor to prevent immune-mediated destruction. 1, 2
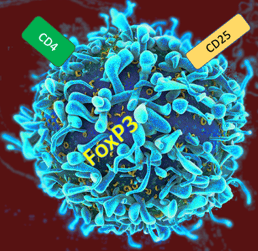
Figure 2: Representation of a regulatory T cell, which expresses the transcription factor FoxP3, as well as CD4 and CD25 as surface markers.
The normal function of a Treg is to maintain immune homeostasis, ensuring that the immune response is measured and not so excessive as to lead to tissue damage or autoimmunity. 2, 3 Tregs are identified with their characteristic expression of CD4 and CD25, as well as the Forkhead box protein 3 (FoxP3) transcription factor. 2, 3 (Figure 2) Adaptations of certain infections or tumor activity can recruit Tregs to prevent the normal immune clearance of pathogens or abnormal cells. 3 Therapeutic development strategies often revolve around attempting to shut off aberrant Treg activity to allow the immune system to perform its duties. 2 Like other immune cells, Treg activity is mediated by cell-cell interactions and soluble factors such as chemokines.
CCR8 as a Therapeutic Target
Originally characterized as an alternative co-receptor for the human immunodeficiency virus (HIV), 4-6 the chemokine receptor CCR8 has now been implicated as a target for antitumor therapies. 7, 8 CCR8 is highly expressed on Treg cells, particularly those associated with tumors in patients with colorectal cancer, 9 melanoma, 10 and many other types of cancer. 7 CCR8 expression is associated with metastatic melanoma, 7 and its preferential expression on Treg cells and its role in immunosuppression makes this immune cell type an attractive mark for novel treatments in both cancer and autoimmune diseases. 8, 11
CCR8 is considered a selective marker for tumor-infiltrating Tregs, and thus there are numerous studies exploring its exploitation in targeted treatment of various cancers. 8, 10 Most of these therapies are based on humanized anti-CCR8 antibodies, which has the effect of depleting Treg cells associated with tumor tissues. 12-14 The ultimate goal of these therapies is to optimize the anti-tumor immune response such that the cytotoxic lymphocytes can destroy the tumor, 15 while preventing the Tregs in the tumor microenvironment from mistakenly suppressing this ability. Whether as a standalone therapy or in combination with other antitumor and immune checkpoint treatments, 14 the importance of CCR8 in ongoing research is evident.
How ABclonal Can Help
ABclonal boasts a wide selection of products for research in cancer and tumor immunology. We are dedicated to supporting your next great discovery that could improve our strategies for treating cancer and improving global health. Please find a sampling of our products below related to surface markers and other features of regulatory T cells.
|
Target |
Cat. No. |
Product |
|
CCR8 |
CCR8 Rabbit mAb |
|
|
CCR8 Rabbit pAb |
||
|
CD4 |
CD4 Rabbit mAb |
|
|
CD4 Rabbit pAb |
||
|
Recombinant Human CD4 Protein |
||
|
Human Cluster of Differentiation 4 ELISA Kit |
||
|
CD25 |
CD25 (Nanobody) Rabbit pAb |
|
|
Recombinant Human CD25/IL-2R alpha Protein |
||
|
Human IL-2R ELISA Kit |
||
|
CD80 |
CD80 Rabbit pAb |
|
|
CD80 Rabbit mAb |
||
|
Recombinant Human B7-1 / CD80 Protein |
||
|
CD86 |
CD86 Rabbit pAb |
|
|
CD86 Rabbit mAb |
||
|
Recombinant Human CD86 Protein |
||
|
Human T-lymphocyte activation antigen CD86 ELISA Kit |
||
|
CTLA4 |
CTLA4 Rabbit pAb |
|
|
Recombinant Human CTLA-4 Protein |
||
|
Human CTLA-4 / CD152 ELISA Kit |
||
|
FOXP3 |
FOXP3 Rabbit pAb |
|
|
FOXP3 Rabbit mAb |
||
|
PD-1 |
PD-1 / CD279 Rabbit pAb |
|
|
Recombinant Human PD-1 Protein |
References
- Hanahan and Weinberg (2011) “Hallmarks of Cancer: The Next Generation.” Cell 144(5):646-674.
- Ohue and Nishikawa (2019) “Regulator T (Treg) cells in cancer: Can Treg cells be a new therapeutic target?” Cancer Sci 110(7):2080-2089.
- Pereira et al. (2017) “Regulatory T Cell and Forkhead Box Protein 3 as Modulators of Immune Homeostasis.” Front Immunol 8:00605 (Epub).
- Lee et al. (2000) “CCR8 on human thymocytes functions as a human immunodeficiency virus type 1 coreceptor.” J Virol 74(15):6946-6952.
- Calado et al. (2010) “Coreceptor usage by HIV-1 and HIV-2 primary isolates: The relevance of CCR8 chemokine receptor as an alternative coreceptor.” Virology 408(2):174-182.
- Santos-Costa et al. (2014) “HIV-2 interaction with cell coreceptors: amino acids within the V1/V2 region of viral envelope are determinant for CCR8, CCR5 and CXCR4 usage.” Retrovirology 11:99 (Epub).
- Korbecki et al. (2020) “CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands.” Int J Mol Sci 21(20):7619.
- Moser B (2022) “Chemokine Receptor-Targeted Therapies: Special Case for CCR8.” Cancers (Basel) 14(3):511 (Epub).
- Villarreal et al. (2018) “Targeting CCR8 Induces Protective Antitumor Immunity and Enhances Vaccine-Induced Responses in Colon Cancer.” Cancer Res 78(18):5340-5348.
- Adams et al. (2021) “Chemokine Pathways in Cutaneous Melanoma: Their Modulation by Cancer and Exploitation by the Clinician.” Cancers (Basel) 13(22):5625 (Epub).
- Barsheshet et al. (2017) “CCR8+ FOXp3+ Treg cells as master drivers of immune regulation.” PNAS 114(23):6086-6091.
- Campbell et al. (2021) “Fc-Optimized Anti-CCR8 Antibody Depletes Regulatory T Cells in Human Tumor Models.” Cancer Res 81(11):2983-2994.
- Kidani et al. (2022) “CCR8-targeted specific depletion of clonally expanded Treg cells in tumor tissues evokes potent tumor immunity with long-lasting memory.” PNAS 119(7):e2114282119 (Epub).
- Van Damme et al. (2021) “Therapeutic depletion of CCR8+ tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy.” J Immunother Cancer 9(2):e001749 (Epub).
- Oliveira et al. (2021) “Phenotype, specificity and avidity of antitumor CD8+ T cells in melanoma.” Nature 596:119-125.