The Problem with Cancer Models
Very few cancer drugs succeed in clinical trials, despite showing promise in the lab. Treatments that may work on animal models, cell lines, or even patient-derived xenografts often do not have the same efficacy in patients. The underlying reason is tumor environments within the human body are far more complex than in research models. For example, the tissue structure (histological complexity) and genetic heterogeneity of an animal model is different than that of humans. Even cell lines and patient-derived xenografts, which are human-derived, have their own pitfalls such as genetic mutations and animal-specific tumor evolution, respectively. Due to the inability to reproduce human tumor environments, many drugs fail clinical trials after lengthy and costly development.
Rise of Organoids
Lately, advances in 3D culture technologies have led to a novel cancer model termed “tumor organoids.” Organoids are miniature 3D replicas of human tissues and organs, grown from primary patient stem cells. In the past year, organoids moved into the spotlight of cancer research because of two important qualities: 1) they highly resemble the tumors that they’re derived from and 2) they are able to predict patient responses to cancer drugs. In a study in Science, researchers recruited 70 colorectal and gastroesophageal cancer patients enrolled in phase 1/2 clinical trials and grew organoids from their biopsies. Comparisons of their phenotypic and genotypic profiling as well as responses to anticancer agents showed that organoids were able to recapitulate real patient responses.
Tumor organoids can be grown with high efficiencies, with the ability to self-organize into organ-like structures. The genetic and phenotypic stability of organoids make them a promising model for human cancers. Scientists have already established long-term organoid cultures from primary colon, oesophagus, pancreas, stomach, liver, endometrium, and breast cancer tissues.
The Future of Cancer
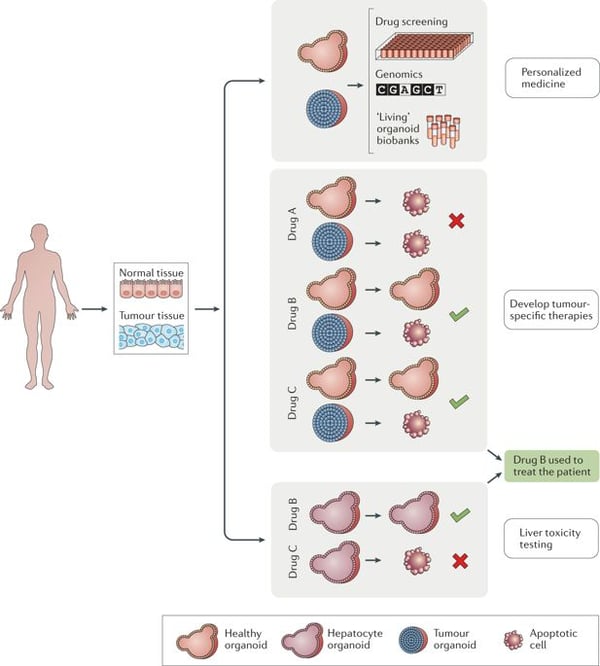
The ability to collect cancer cells from patients and grow them into mini tumors for drug testing may be ground-breaking for drug development and personalized medicine. At the annual ASCB (American Society for Cell Biology) meeting last December, the Soragni lab presented their work on tumor organoids. As reported by Science News, Soragni says that tumor organoids could open a whole new arena for patients with rare cancers. By growing primary-patient derived tumor organoids, researchers can test different therapies and combinations of drugs to create personalized treatment plans that work. For example, Soragni’s group tested 430 compounds on organoids grown from the cells of a boy with a rare bone cancer. Tumor organoids may be an efficient model for cancer research and promote the translation of basic cancer research to clinical practice. There will likely be a significant rise in tumor organoid models in 2019.

.jpg)