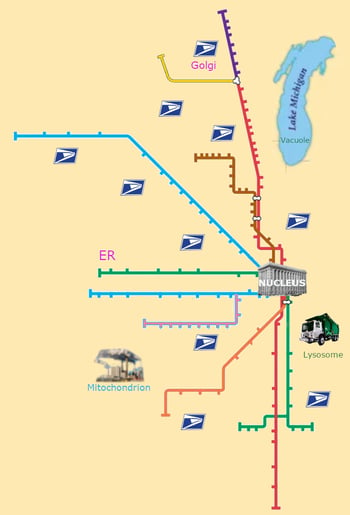
When I taught high school biology, a favorite part of the curriculum was cellular structures and functions. I set up an activity suggested by other experienced biology teachers that was based on the “Cell City,” a learning analogy where students would create an artwork of a city with the mitochondrion as a power plant and a vacuole as a lake. (Figure 1) I wish I saved their very creative projects, but I distinctly remember one group used the Chicago Transit Authority’s elevated train system map to represent the endoplasmic reticulum (ER), a very clever use of the analogy and a nod to city pride. It was also the first time these students really thought about vesicular transport, although they didn't fully understand its importance.
I met Randy Schekman as an undergraduate at the University of California at Berkeley, when he capably demonstrated membrane transport and aquaporins in our cell biology course. Later, I realized that he was revered for his work on the Golgi apparatus. While in graduate school, I learned more intricate details about the vesicular transport system from a professor who came from James Rothman’s laboratory (a fantastic teacher whose style I tried to emulate), and I got to appreciate the beauty and functional significance of the ER, Golgi, and the vesicular transport system in normal health and disease.
Figure 1 (above right): The “Cell City” analogy of cellular organelles and compartmentalization, featuring Chicago’s City Hall (nucleus) surrounded by the CTA train system (ER). Adapted from something some of my students did many moons ago (Lake Michigan not to scale).
In addition to their contributions to higher education (which inspired me to become a teacher), Schekman, Rothman, and Thomas Sudhof were awarded the 2013 Nobel Prize in Physiology or Medicine for elucidating the mechanisms of vesicular transport. This well-deserved recognition underlies how disruptions to cellular trafficking contribute to many diseases and disorders.
Cellular Delivery Service: Normal Functions in Vesicular Transport
Following Camillo Golgi's description of the eponymous cellular structures in 1898, the advances in understanding the principles of membrane-bound organelle-based trafficking pathways have been crucial in clarifying the role these structures play in maintaining normal health and how they contribute to various human diseases. 1, 2 (Figure 2) The ability of the cell to transport molecular cargo to their exact subcellular compartment in a timely fashion is key to homeostasis. Through a beautifully complex coordination between numerous protein markers, receptors, and other factors, the transport of properly folded proteins follows a tightly regulated series of procedures: 2
- Selection of protein cargo, assisted by adaptors;
- Encapsulation of protein cargo within a membrane-bound vesicle;
- Movement of the vesicle from its initial compartment to a target compartment;
- Tethering the vesicle to the target compartment membrane;
- Docking and membrane fusion to deliver protein cargo to the target compartment.
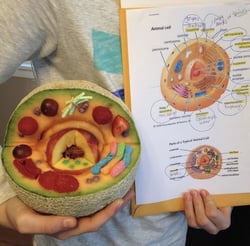
Figure 2 (right): A representation of an animal cell, featuring the Golgi apparatus (Fruit Roll-Ups) and the ER (Gummi Worms). Source: Twitter.
Upon initial synthesis by cytosolic ribosomes, nascent proteins intended to be secreted or integrated in a membrane are recognized and directed to the ER, where protein folding is assisted by chaperone proteins such as heat shock protein 90 (HSP90). Properly folded proteins are packaged and shuttled to the Golgi apparatus through the action of coat protein complex (COP) II vesicles and clathrin. Traffic also flows from the Golgi back to the ER using COP I vesicles to recycle sorting proteins. 2-4 The Golgi further modifies and packages protein cargo in vesicles destined for either the plasma membrane or an endosomal compartment such as a lysosome. 2
The ER performs a crucial checkpoint termed the unfolded protein response (UPR) to detect stress due to improperly folded or unfolded proteins that could accumulate and disrupt normal cellular functions. 3 UPR refolds proteins if possible, and otherwise marks them for ER-associated degradation (ERAD) involving ubiquitination and the proteasome. 2, 4-6 If UPR cannot restore homeostasis, the cell will induce autophagy or cell death through PERK, IRE1, and ATF6. 3, 5, 6 Golgi apparatus related degradation (GARD) also feeds into ERAD in response to stress stimuli, and endosome and Golgi-associated degradation (EGAD) can bypass ERAD to direct faulty proteins to cytosolic proteasomes. 1 Maintaining the balance of properly folded proteins is essential for normal cellular function and survival.
Pile Up: When Bad Proteins Accumulate
Vesicular transport checkpoints are usually effective at regulating cellular homeostasis and protein content (proteostasis). Cells undergoing stress unable to trigger proper UPR may evade cell death, leading to diseases that include neurological disorders, muscular dystrophy, Crohn’s disease, diabetes, and cancer. 2, 4, 5
Excesses of misfolded proteins, such as tau or beta-amyloid, in the ER are associated with neuronal death and neurological disorders. 5, 6 In neurons, defective proteins are detected by GRP78, or bind directly to PERK and IRE1. Inappropriate activation of UPR triggers cell death through different mechanisms, including apoptosis, autophagic cell death, and necroptosis (a necrotic cell death mediated by receptor interacting protein kinases RIPK1, RIPK3, and the mixed lineage kinase domain-like protein MLKL). 6 Prolonged ER stress and Golgi-related abnormalities in the central nervous system can also lead to neuroinflammation and autoimmune diseases such as multiple sclerosis. 5, 6
Protein trafficking disruptions are also linked to metabolic disorders and diabetes. Abnormal metabolism is also linked to cancers and exacerbated by ER- and Golgi-related stress. 4, 5, 7, 8 Increased expression of the HSP90 isoform GRP94 may allow stabilization of various tumorigenic proteins in cancer cells. 4 The Golgi performs key glycosylation to cancer proteins, promotes the DNA damage response to enhance cell survival, and stimulates p38-mediated and mTOR signaling. 7 Remarkably, the Golgi apparatus can fragment or change morphology to orient at the leading edge to promote formation of invasive structures like filopodia, while promoting the breakdown of extracellular matrix, allowing for migration and metastasis. 7, 8 Pathogens like viruses, including the SARS-CoV-2 coronavirus, specifically disrupt the Golgi and subsequently appropriate and fragment host ER and Golgi membranes to replicate new virus particles. 9 (Figure 3) The devoted efforts to examining the vesicular transport system in normal physiology and disease are vital to our collective health.
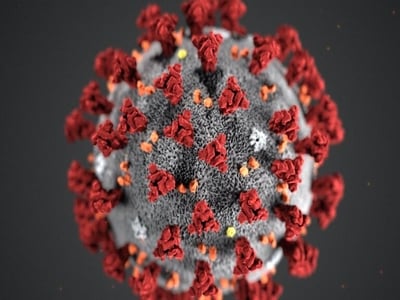
Figure 3: A typical coronavirus structure, with host membrane markers often found within the viral coat.
Clearing Traffic Jams: Therapeutic Opportunities
Much of how cells use the ER and Golgi to target cargo throughout the cell is still unclear, making it difficult to generate novel therapies to leverage these organelles in associated diseases. However, some promising advances in research and therapeutic development have been made.
 In ocular diseases such as retinal dystrophy choroideremia, mutated RAB escort protein 1 (REP-1) disrupts membrane trafficking. Recently, a viral delivery vector was developed to administer the REP-1 gene directly to the eye, with Phase III clinical trials underway. 2 The study by Ghosh et al. knocked down GRP94 and decreased cell migration and adhesion due to a defect in vesicular transport, suggesting a potential therapeutic target against aggressive cancers. 4 Therapies to improve ER homeostasis in diabetes patients have been explored to target chaperone molecules, and to directly modulate PERK and IRE1 to reduce the inflammatory response. 5 Various Golgi markers, such as GRASP55 and GM130, are overexpressed in cancers, making them intriguing drug targets. 8
In ocular diseases such as retinal dystrophy choroideremia, mutated RAB escort protein 1 (REP-1) disrupts membrane trafficking. Recently, a viral delivery vector was developed to administer the REP-1 gene directly to the eye, with Phase III clinical trials underway. 2 The study by Ghosh et al. knocked down GRP94 and decreased cell migration and adhesion due to a defect in vesicular transport, suggesting a potential therapeutic target against aggressive cancers. 4 Therapies to improve ER homeostasis in diabetes patients have been explored to target chaperone molecules, and to directly modulate PERK and IRE1 to reduce the inflammatory response. 5 Various Golgi markers, such as GRASP55 and GM130, are overexpressed in cancers, making them intriguing drug targets. 8
A rapidly growing field is in extracellular vesicle (EV) biology, particularly focused on exosomes. First discovered and characterized in the 1980s, exosomes are small EVs that serve as intercellular messengers, originating from a parent cell to transmit cargo and signals to a target cell. 10-14 Exosomes derived from tumor cells hold nucleic acids of all types, including prognostic microRNAs, and proteins that are being studied for their utility as early detection biomarkers. 10, 11 Exosome biogenesis is also commandeered by viruses that have infected the host cell, which allows them to continue spreading infection and to evade the immune system. 12 EVs, including exosomes, have been known to promote neural development by sending protein cargo such as cyclin D1 to target cells, and to eliminate wastes and damaged proteins from neurons. 11, 13, 14 Exosomal transport of damaged cellular material could accumulate throughout the central nervous system and promote neurodegeneration, making exosomes key biomarkers in the early clinical diagnosis of these disorders. 11, 14 Since exosomes are enriched in surface markers such as CD9, CD63, and CD81, they can usually be easily identified, albeit not as easily purified. 11
Targeted therapies against endosomal and exosomal factors are technically difficult due to the issues in isolating and enriching the desired molecules and vesicles. 2, 10, 14 Despite the inherent challenges, there is hope that with advancing technologies and a deeper understanding of vesicular transport, medical science can one day leverage these therapeutic strategies to reduce the burden of neurodegenerative disorders and cancer, among many other applications to continue promoting human health.
How ABclonal Can Help
ABclonal is pleased to continue working with and supporting passionate scientists like you in further dissecting vesicular transport pathways. Our product lines are validated by researchers in scientific publications, and we want to be your partner in designing new experimental strategies to isolate and characterize components within the pathway. You can browse catalog antibody products to the various targets linked above, and reagents to study selected ER, Golgi, and vesicular markers below.
|
Target |
Cat. No. |
Product Description |
|
Calnexin |
Calnexin Rabbit mAb |
|
|
Calnexin Rabbit pAb |
||
|
Human Calnexin ELISA Kit (CNX) |
||
|
Calreticulin |
Calreticulin Rabbit pAb |
|
|
COPA |
COPA Rabbit mAb |
|
|
COPA Rabbit pAb |
||
|
GOLPH2 / GP73 |
GOLPH2 Rabbit mAb |
|
|
GOLPH2 Rabbit pAb |
||
|
Human Golgi Protein 73 ELISA Kit (GP73) |
||
|
SERCA2 |
SERCA2 / ATP2A2 Rabbit mAb |
|
|
SERCA2 / ATP2A2 Rabbit pAb |
||
|
Phospho-SERCA2 / ATP2A2 – Thr484 Rabbit pAb |
||
|
SYT1 |
SYT1 Rabbit pAb |
|
|
UGGT1 |
UGGT1 Rabbit pAb |
|
|
USO1 |
USO1 Rabbit mAb |
|
|
USO1 Rabbit pAb |
||
|
VAMP1 |
VAMP1 Rabbit pAb |
|
|
VAMP2 |
VAMP2 Rabbit mAb |
|
|
VAPA |
VAPA Rabbit pAb |
|
|
VAPB |
VAPB Rabbit pAb |
References
- Benyair et al. (2022) “Maintaining Golgi Homeostasis: A balancing Act of Two Proteolytic Pathways.” Cells 11(5):780 (Epub).
- Yarwood et al. (2020) “Membrane trafficking in health and disease.” Dis Model Mech 13(4):dmm043448 (Epub).
- Almanza et al. (2019) “Endoplasmic reticulum stress signalling – from basic mechanisms to clinical applications.” FEBS J 286(2):241-278.
- Ghosh et al. (2016) “Endoplasmic Reticulum-resident Heat Shock Protein 90 (HSP90) Isoform Glucose-regulated Protein 94 (GRP94) Regulates Cell Polarity and Cancer Cell Migration by Affecting Intracellular Transport.” J Biol Chem 291(16):8309-8323.
- Mustapha et al. (2021) “Current Status of Endoplasmic Reticulum Stress in Type II Diabetes.” Molecules 26(14):4362 (Epub).
- Shi et al. (2021) “Endoplasmic Reticulum Stress-Associated Neuronal Death and Innate Immune Response in Neurological Diseases.” Front Immunol 12:794580 (Epub).
- Liu et al. (2021) “The role of the Golgi apparatus in disease (Review).” Int J Mol Med 47(4):38 (Epub).
- Bui et al. (2021) “Adaptation of the Golgi Apparatus in Cancer Cell Invasion and Metastasis.” Front Cell Dev Biol 9:806482 (Epub).
- Hackstadt et al. (2021) “Disruption of the Golgi Apparatus and Contribution of the Endoplasmic Reticulum to the SARS-CoV-2 Replication Complex.” Viruses 13(9):1798 (Epub).
- Saad et al. (2021) “Exosomes as Powerful Engines in Cancer: Isolation, Characterization and Detection Techniques.” Biosensors (Basel) 11(12):518 (Epub).
- Perocheau et al. (2021) “Clinical applications for exosomes: Are we there yet?” Br J Pharmacol 178(12):2375-2392.
- Chaudhari et al. (2022) “Multifunctional role of exosomes in viral diseases: From transmission to diagnosis and therapy.” Cell Signal 94:110325 (Epub).
- Song et al. (2021) “Extracellular vesicles from neurons promote neural induction of stem cells through cyclin D1.” J Cell Biol 220(9):e202101075 (Epub).
- Beatriz et al. (2021) “Exosomes: Innocent Bystanders or Critical Culprits in Neurodegenerative Diseases.” Front Cell Dev Biol 9:635104 (Epub).