 Once upon a time when I was a fledgling science nerd in high school, I started learning about the process of apoptosis, which remains to this day the most studied form of cell death in various functions including organismal development and defense against cancer. As an immunologist-in-training, I also learned about the classical complement pathway that the immune system uses to destroy infected cells, and also necrotic cell death or necroptosis (which is full of really gross pictures if you dare to Google it). Of course, I learned about autophagy in graduate school and really appreciate its utility in normal physiology and disease, while very recently I read about ferroptosis as yet another programmed cell death (PCD) pathway. Right around when the Nobel Prize was awarded to recognize the elucidation of PCD, pyroptosis came about as a novel PCD pathway that is continuing to gain steam in its clinical relevance. It seems logical for cells and organisms to have redundant systems in place to clear away damaged and malignant cells before a health crisis can emerge if the cell evades the primary route of apoptosis.
Once upon a time when I was a fledgling science nerd in high school, I started learning about the process of apoptosis, which remains to this day the most studied form of cell death in various functions including organismal development and defense against cancer. As an immunologist-in-training, I also learned about the classical complement pathway that the immune system uses to destroy infected cells, and also necrotic cell death or necroptosis (which is full of really gross pictures if you dare to Google it). Of course, I learned about autophagy in graduate school and really appreciate its utility in normal physiology and disease, while very recently I read about ferroptosis as yet another programmed cell death (PCD) pathway. Right around when the Nobel Prize was awarded to recognize the elucidation of PCD, pyroptosis came about as a novel PCD pathway that is continuing to gain steam in its clinical relevance. It seems logical for cells and organisms to have redundant systems in place to clear away damaged and malignant cells before a health crisis can emerge if the cell evades the primary route of apoptosis.
The Flourishing Field of Pyroptosis
First described by Brad Cookson and Molly Brennan in 2001, the term “pyroptosis” derives from Greek “pyro” meaning “fire” and “ptosis” meaning falling, referring to the proinflammatory signaling emanating from the dying cell. A data mining study for the two decades after Cookson and Brennan’s first report revealed over 2800 publications with annual increases in total publications. 1 Confirming what I noticed while researching pyroptosis on Pubmed, the relevant publications came from 70 countries led by China, with many of the reviews from Chinese authors. 1 The data mining study also revealed a common keywords cloud that included the key factors in triggering pyroptosis, including gasdermin D (GSDMD). 1 As is sometimes the case, whenever Penny asks me about a random protein or gene (in this case, GSDMD), I’m able to tell her what it is, but then my curiosity takes over and I learn a lot more about the drug target and its pathways, so let’s share what I learned about pyroptosis!
Pyroptosis in Host Defense
PCD, including pyroptosis, plays critical roles in fighting infections and injury and in maintaining organismal homeostasis. 2 Pyroptosis is considered an inflammatory form of necrosis that is dependent on caspase activation. The hallmark of pyroptosis is the formation of pores in the cell membrane that leads to cell lysis and the release of proinflammatory cytokines. 2 The consensus is that there are three pathways driving pyroptosis, (Figure 1) which include:
- The canonical inflammasome pathway (Caspase-1-dependent)
- The noncanonical inflammasome pathway (Caspase-1-independent)
- Caspase-8 / -3 / Gasdermin E (GSDME) pathway 2, 3
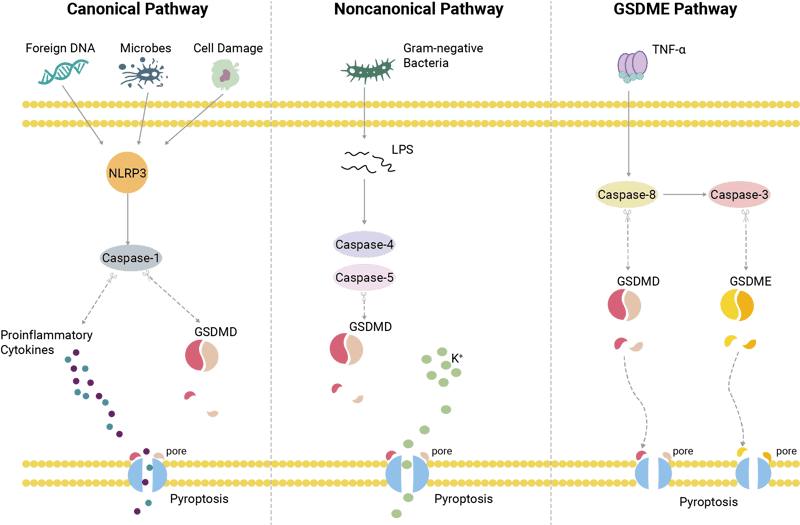
Figure 1: The three major pyroptosis pathways that culminate in gasdermin-mediated pore formation in the cell membrane.
The term “inflammasome” refers to the cytosolic sensor molecules that detect foreign matter. 4 The canonical pathway uses inflammasome sensor proteins such as NLRP3 to detect antigens and recruit the adaptor protein ASC to activate caspase-1. Subsequently, caspase-1 will cleave GSDMD which then forms pores in the cell membrane. Caspase-1 also produces mature IL-1beta and IL-18 to be released upon cell lysis and initiate the inflammatory response. The noncanonical pathway uses lipopolysaccharide (LPS) to activate other caspases that in turn activate GSDMD similarly to the canonical pathway. Finally, the GSDME pathway is activated by TNF-alpha, which acts through caspase-8 and caspase-3. 2 (Figure 1) Other proinflammatory caspases that are linked to pyroptosis include caspase-4, caspase-5, and caspase-11. 3
The primary function of pyroptosis to combat bacterial infections is very intriguing, as the mechanism seems to only kill the infected host cell and not the bacteria. 4 Instead, the pyroptotic cell traps the still-alive bacteria in a structure known as the “pore-induced intracellular trap” (PIT) which immobilizes the bacteria while recruiting innate immune cells such as neutrophils to kill the bacteria via phagocytosis. 4
Key Players in Pyroptosis
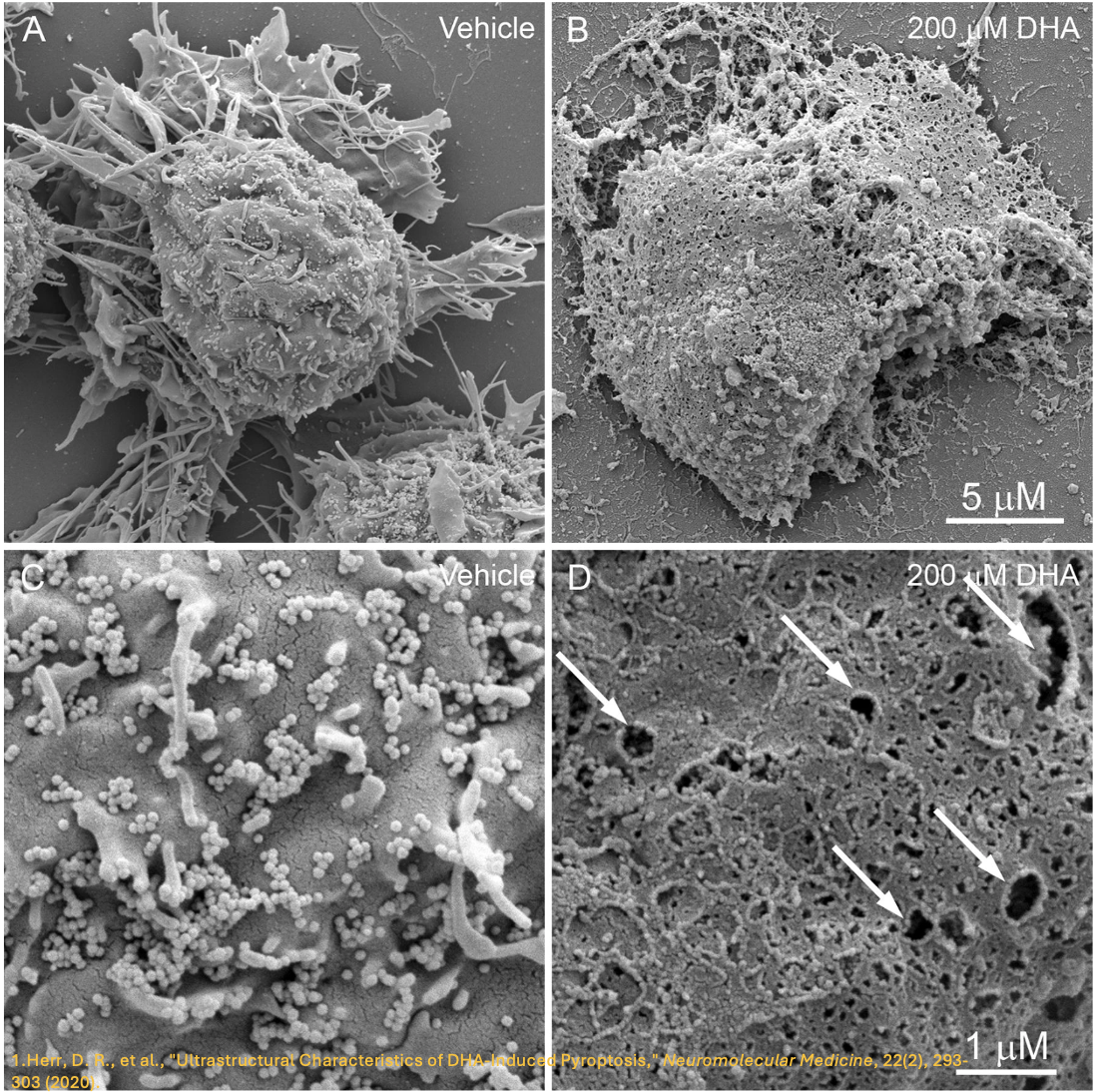
While other gasdermins (including Gasdermin C 5) can be activated through caspase-mediated pathways to induce pyroptosis, the predominant gasdermin studied in this field is GSDMD, identified in 2015 as responsible for pyroptosis in immune cells. 2-4, 6, 7 GSDMD is expressed in immune cells, the placenta, gastrointestinal epithelium, and many types of cancer. 7 Cleavage of GSDMD at aspartate 276 by caspase-1, -4, or -5 in humans releases an amino-terminal fragment that assembles pores in the plasma membrane to effect pyroptosis, comparable to how the classical complement pathway forms a membrane attack complex to kill the cell. 6 (Figure 2) GSDMD pores are large enough for cytokines to escape the cell, but not large enough for larger proteins such as lactate dehydrogenase (LDH), which is why assaying the release of LDH is useful as a marker for when an immune cell is hyperactivated but not pyroptotic. Hyperactivated cells can sustain the inflammatory response since they survive. 7 Activated GSDMD is also known to bind to mitochondrial membranes to cause damage. 7
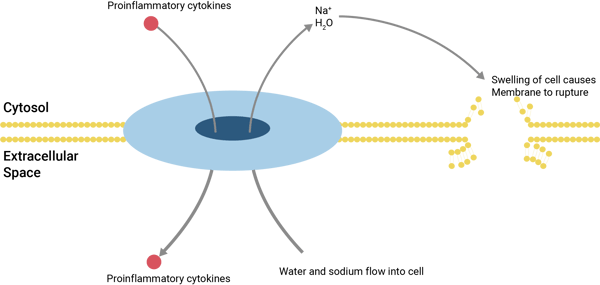
Figure 2: Gasdermin units assemble within the plasma membrane to form a pore that allows the efflux of proinflammatory cytokines to sustain inflammation. The pores also allow the influx of ions and water to swell the cell and eventually cause the membrane to rupture, leading to cell lysis and death.
One of the key components that assembles the inflammasome complex is the Nod-like receptor (NLR) family pyrin domain-containing proteins, which includes NLRP3. 2-4, 6-9 NLRP3 inflammasomes are activated by many different stimuli, and can be activated in response to a decrease in intracellular potassium concentration, which alters the conformation and allows oligomerization of NLRP3. 8 It was recently reported that a critical mediator of NLRP3 activation and its ability to assemble the inflammasome complex following stimulation by LPS is the phosphorylation of its leucine-rich repeat (LRR) domain at serine 803 by the kinase CSNK1A1. 9
The Unfortunate Disease Potential of Pyroptosis
Despite its critical role in the detection of foreign matter and driving innate immunity, pyroptosis and pyroptosis-mediated inflammation is implicated in many diseases, including periodontitis, 10 autoimmune disorders such as rheumatoid arthritis, 11 and contributes to disease progression for both viral (such as Hepatitis C) 12 and bacterial infections (including Staphylococcus aureus). 13
In a bit of a paradox, pyroptosis may both impair and enhance the persistence of diseases, including cancer. As a form of PCD, pyroptosis would logically prevent tumor development, but the inflammatory signals released due to pyroptosis could also drive tumorigenesis. 14, 15 There are some expression patterns of core pyroptosis proteins that are correlated with various cancers, including the low expression level of NLRP3 in hepatocellular carcinoma and colorectal cancers. 14 By contrast, there are different outcomes from GSDMD expression levels in the progression of colorectal or lung cancer, where GSDMD is highly expressed, versus gastric cancer where GSDMD is downregulated. 14
Therapeutic Strategies to Manipulate Pyroptosis
 Delineating the expression profiles of pyroptosis factors may be key to developing new strategies that aim to modulate pyroptosis to prevent tumor growth. 14, 15 In clear cell renal cell carcinoma, the overexpression of the inflammasome sensor AIM2 is correlated to tumor progression and can be a predictor of responsiveness to immunotherapy. 15 Treatment strategies include small molecule inhibitors and immunotherapies aimed at inflammasome sensors such as NLRP3, and the gasdermins to control the formation of pores, either inhibiting or promoting pyroptosis depending on the type and grade of cancer. 16-18 A large swath of research is also dedicated to the use of non-coding RNA, including microRNA, to downregulate initiators of pyroptosis, including caspase-1. 14, 16, 19
Delineating the expression profiles of pyroptosis factors may be key to developing new strategies that aim to modulate pyroptosis to prevent tumor growth. 14, 15 In clear cell renal cell carcinoma, the overexpression of the inflammasome sensor AIM2 is correlated to tumor progression and can be a predictor of responsiveness to immunotherapy. 15 Treatment strategies include small molecule inhibitors and immunotherapies aimed at inflammasome sensors such as NLRP3, and the gasdermins to control the formation of pores, either inhibiting or promoting pyroptosis depending on the type and grade of cancer. 16-18 A large swath of research is also dedicated to the use of non-coding RNA, including microRNA, to downregulate initiators of pyroptosis, including caspase-1. 14, 16, 19
How ABclonal Can Help
Throughout this article, I have hyperlinked the core pyroptosis targets to ABclonal antibody product pages. ABclonal also provides ELISA kits to detect your desired target, including for human GSDMD. ABclonal has a proven track record of success in pyroptosis research, backed by customer feedback and scientific publications. Due to the interplay between pyroptosis and other PCD pathways, please also find the following product categories, and feel free to reach out if we can help you design your study or find the appropriate products!
References
- Ma et al. (2021) “A Bibliometric Analysis of Pyroptosis from 2001 to 2021.” Front Immunol 12:731933 (Epub).
- Wu et al. (2022) “Cell pyroptosis in health and inflammatory diseases.” Cell Death Discov 8:191 (Epub).
- Kesavardhana et al. (2020) “Caspases in Cell Death, Inflammation, and Gasdermin-Induced Pyroptosis.” Annu Rev Immunol 38:567-595.
- Kovacs & Miao (2017) “Gasdermins: Effectors of pyroptosis.” Trends Cell Biol 27(9):673-684.
- Zhang et al. (2021) “The metabolite a-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8.” Cell Res 31(9):980-997.
- de Vasconcelos & Lamkanfi (2020) “Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis.” Cold Spring Harb Perspect Biol 12(5):a036392 (Epub).
- Liu et al. (2021) “Channelling inflammation: gasdermins in physiology and disease.” Nat Rev Drug Discov 20(5):384-405.
- Tapia-Abellán et al. (2021) “Sensing low intracellular potassium by NLRP3 results in a stable open structure that promotes inflammasome activation.” Sci Adv 7(38):eabf4468 (Epub).
- Niu et al. (2021) “NLRP3 phosphorylation in its LRR domain critically regulates inflammasome assembly.” Nat Commun 12:5862 (Epub).
- Sordi et al. (2022) “Pyroptosis-Mediated Periodontal Disease.” Int J Mol Sci 23(1):372 (Epub).
- Demarco et al. (2022) “How Pyroptosis Contributes to Inflammation and Fibroblast-Macrophage Cross-Talk in Rheumatoid Arthritis.” Cells 11(8):1307 (Epub).
- Wallace et al. (2022) “Crosstalk Between Pyroptosis and Apoptosis in Hepatitis C Virus-induced Cell Death.” Front Immunol 13:788138 (Epub).
- Pastar et al. (2021) “Intracellular Staphylococcus aureus triggers pyroptosis and contributes to inhibition of healing due to perforin-2 suppression.” J Clin Invest 131(24):e133727 (Epub).
- Xia et al. (2019) “The role of pyroptosis in cancer: pro-cancer or pro-‘host’?” Cell Death Dis 10(9):650 (Epub).
- Zhang et al. (2021) “Pyroptosis Regulators and Tumor Microenvironment Infiltration Characterization in Clear Cell Renal Cell Carcinoma.” Front Oncol 11:774279 (Epub).
- Hsu et al. (2021) “Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment.” Theranostics 11(18):8813-8835.
- Fischer et al. (2021) “Posttranslational and Therapeutic Control of Gasdermin-Mediated Pyroptosis and Inflammation.” Front Immunol 12:661162 (Epub).
- Missiroli et al. (2021) “Targeting the NLRP3 Inflammasome as a New Therapeutic Option for Overcoming Cancer.” Cancers (Basel) 13(10):2297 (Epub).
- Gao et al. (2022) “Regulation of Pyroptosis by ncRNA: A Novel Research Direction.” Front Cell Dev Biol 10:840576 (Epub).