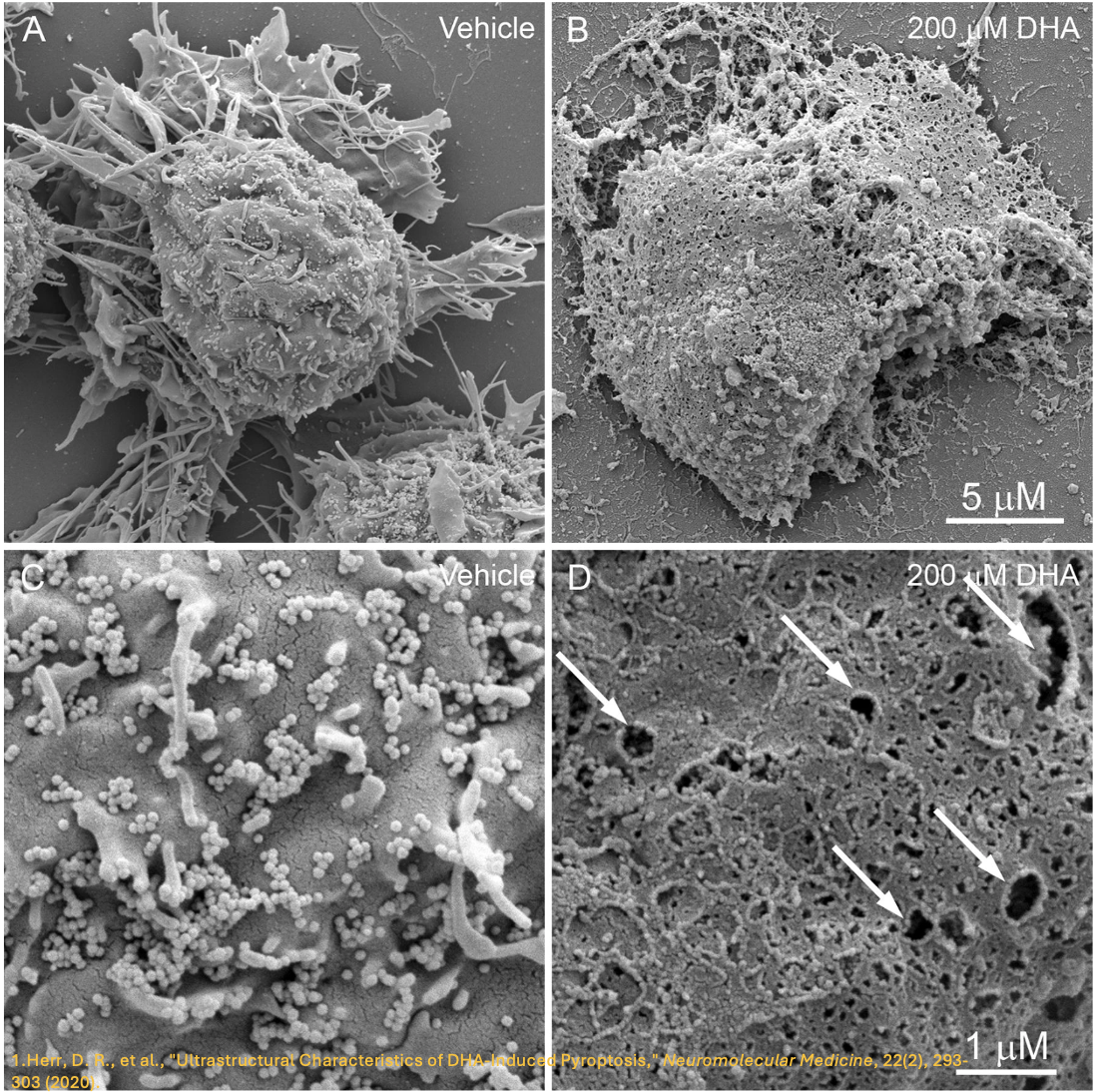
It is evident that the gasdermin family is central to pyroptosis. This is because the gasdermin family members are the executioners of pyroptosis, and their pore-forming function is a necessary condition for pyroptosis to occur. This family generally includes a cytotoxic N-terminal domain and a C-terminal inhibitory domain. After cleavage, the N-terminal domain is released and can assemble into pores in the membrane. Gasdermin pores disrupt the integrity of the cell membrane, leading to inflammatory cell death, with cell contents, including inflammatory cytokines, being released into the extracellular space.