 Every now and then when I get hungry, I joke that my stomach is about to digest itself. For the longest time, human science was unaware that our cells could literally eat itself (or more precisely, parts of itself) as well! First described in the 1960s by Christian de Duve (who won the Nobel Prize for discovering the lysosome), the term autophagy derives from Greek words combined to mean “self-eating” and describes a process by which the cell degrades large components and organelles in a distinct mechanism. 1-3 The phenomenon was not studied extensively until the 1990s, when Yoshinori Ohsumi performed a series of groundbreaking experiments to determine the underlying mechanisms of autophagy, an achievement for which he was awarded the 2016 Nobel Prize in Physiology or Medicine. Ohsumi’s work has led to an explosion of research that has precipitated a greater understanding of the role played by cellular digestion, degradation, and recycling pathways in human health and disease.
Every now and then when I get hungry, I joke that my stomach is about to digest itself. For the longest time, human science was unaware that our cells could literally eat itself (or more precisely, parts of itself) as well! First described in the 1960s by Christian de Duve (who won the Nobel Prize for discovering the lysosome), the term autophagy derives from Greek words combined to mean “self-eating” and describes a process by which the cell degrades large components and organelles in a distinct mechanism. 1-3 The phenomenon was not studied extensively until the 1990s, when Yoshinori Ohsumi performed a series of groundbreaking experiments to determine the underlying mechanisms of autophagy, an achievement for which he was awarded the 2016 Nobel Prize in Physiology or Medicine. Ohsumi’s work has led to an explosion of research that has precipitated a greater understanding of the role played by cellular digestion, degradation, and recycling pathways in human health and disease.
Editor’s note (and by editor, I mean me): We are starting a regular feature on the ABclonal blog we would like to call “ABclonal in Action,” where we will highlight products from our catalog that have been used in research published in scientific journals. Probably should have started this a while back, but just like scientific ideas, you never know when inspiration will strike. Enjoy!
The Sin of Gluttony: Autophagy in Disease
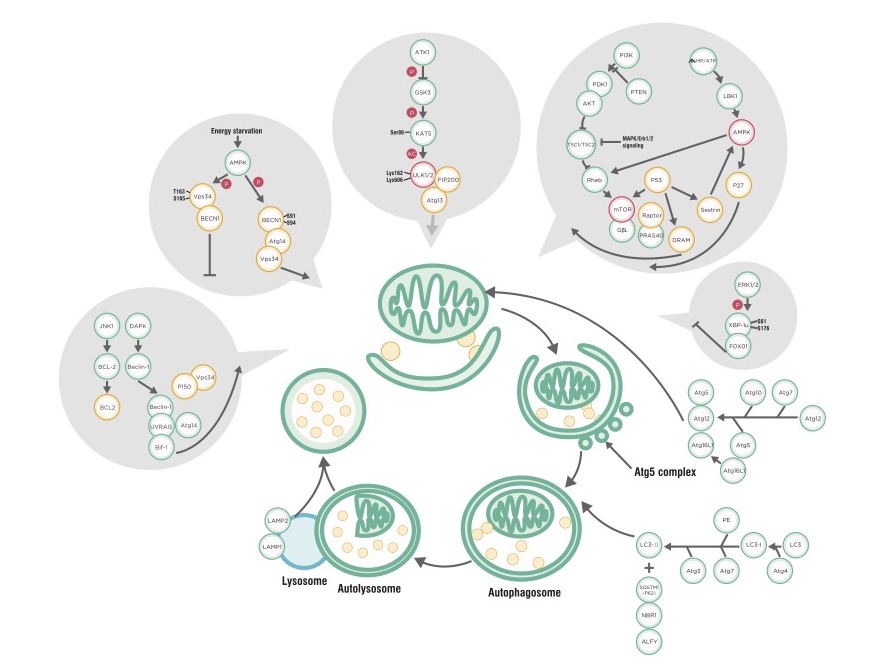
It seemed that autophagy was a nascent field when I started graduate school, as I never heard the term until I took a cancer biology class with Kay Macleod, who was the foremost authority on autophagy on campus. You can read about the general mechanism of autophagy here, but the basic function is to direct intracellular components to a digestive organelle (either the lysosome or an autophagosome) for degradation and recycling. 4 The autophagy pathways are intricately tied into key cellular functions including stress detection, metabolism, and even cell death, underlying the importance of further studying this process and its contributions to disease. 4
Autophagy and membrane-bound vesicles go hand in hand, and drive many of the same types of disorders across the spectrum due to their connection to nearly all cellular functions. Mutations and excess accumulation of the ubiquitin-binding protein SQSTM1, which also interacts with several autophagy-related proteins, is highly correlated with neurodegenerative disorders such as Parkinson’s disease and amyotrophic lateral sclerosis (ALS). 1, 2 Defects in autophagic signaling and associated processes can lead to cardiovascular diseases and various organ failures, as well as metabolic syndromes including diabetes. 4 Perhaps the most prevalent field in autophagy research focuses on its complex role in cancer, whether to promote tumor growth or as a checkpoint against cancer progression. 3, 4 Decoding the convoluted mechanisms of autophagy would logically lead to new insights into more effective targeted therapies against numerous human ailments.
Leveraging Autophagy to Combat Disease
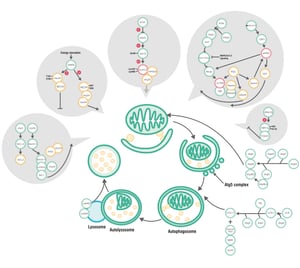 The difficulty in targeting autophagy as a remedy is that this process occurs in virtually every cell type in the body at different rates dependent on many organismal and environmental conditions. Therefore, the challenge lies in developing cell-type-specific model systems that are relevant to the disease state of interest, and that requires a deeper understanding of how autophagy is initiated and subsequently functions in, for example, a nerve cell versus a kidney cell. 1 While the cancer therapy strategy is to block autophagy due to its reported pro-tumorigenic activities, in neurodegenerative disorders, the consensus is to stimulate autophagic pathways instead. 2, 3
The difficulty in targeting autophagy as a remedy is that this process occurs in virtually every cell type in the body at different rates dependent on many organismal and environmental conditions. Therefore, the challenge lies in developing cell-type-specific model systems that are relevant to the disease state of interest, and that requires a deeper understanding of how autophagy is initiated and subsequently functions in, for example, a nerve cell versus a kidney cell. 1 While the cancer therapy strategy is to block autophagy due to its reported pro-tumorigenic activities, in neurodegenerative disorders, the consensus is to stimulate autophagic pathways instead. 2, 3
Inhibition of autophagy-related genes (ATG) such as isoforms of ATG4 (which processes LC3), or ULK1, a major autophagy regulator, have been explored as potential targets in cancer therapy. 3 Directly targeting the autophagosome system in concert with lysosome inhibitors has also been attempted. 3, 5 Modulation of autophagy in cancer cell models show that activating and inhibiting the process at various phases of cancer progression has some efficacy. For example, due to the disruption of normal autophagy in early stages of cancer, a stimulation strategy would be more appropriate, while at late stages, autophagy sustains tumor growth. 3, 5 Unique strategies depending on the characteristics and grade of cancer being treated would therefore be necessary.
With advances in drug design and screening, many more small molecules have been added to the repertoire of autophagy modulating agents. A large portion of these strategies aim at the manipulation of pathways upstream of autophagy such as the AMPK pathway or the mTOR pathway. 2, 6 Other agents that can inhibit the fusion of autophagosomes with lysosomes can sensitize cancer cells to antitumor therapies. 5, 6 Selectively targeting autophagosome lipids can also modulate the severity of symptoms related to autophagy. 6 Unfortunately, due to toxicity and autophagy-independent off-target effects, many of these strategies are not currently viable in the clinic. 5-7
Working Toward a Better Understanding of Autophagy
 Every new study that is announced in a news release or published in a scientific journal, no matter its impact factor, adds to our growing knowledge of the factors and functions of new cell- and context-specific components of autophagy. I am pleased to see that ABclonal is able to support this continuing quest to understand human physiology, and there are many more examples than this in the current literature showing our products in action.
Every new study that is announced in a news release or published in a scientific journal, no matter its impact factor, adds to our growing knowledge of the factors and functions of new cell- and context-specific components of autophagy. I am pleased to see that ABclonal is able to support this continuing quest to understand human physiology, and there are many more examples than this in the current literature showing our products in action.
In a study of lung adenocarcinoma, Ying Liu and Yun Du of the Hebei Medical University in China showed that lung cancer cell lines resistant to EGFR tyrosine kinase inhibitors could still be abated through inhibition of autophagy. 8 By knocking down ATG5, autophagy was suppressed and led to an impairment in the migration and invasive ability of the cells tested, corroborating findings that autophagy was a pro-tumorigenic process in lung cancer. ABclonal’s SQSTM1/p62 Rabbit Polyclonal Antibody was used in this study to track autophagy, among other targets related to migration and invasion of cancer cells.
In a separate study, a group from the University of Tokyo in Japan studied the role of autophagy in regulating the formation of primary cilia, microtubule-based organelles that extend from the cell membrane. Based on reports that autophagy and the generation of cilia is codependent, the group was able to show that the NIMA-related kinase 9 (NEK9) and its LC3-interacting region (LIR) are required to produce primary cilia, and defects in this interaction impairs cilia formation. 9 From ABclonal, this study used antibody products targeting NEK8, MYH9, and MYH10 to further elaborate on the mechanism of cilia formation.
How ABclonal Can Help
ABclonal is dedicated to supporting the next breakthrough in autophagy research. Please browse our products related to the study of autophagy and its upstream and downstream pathways. I have also linked several of our antibodies to the text of this blog. If you need help finding a more suitable reagent or want to know more about how to best apply our products to your experimental platforms, please reach out and we would be delighted to assist!
References
- Chua et al. (2022) “Autophagy and ALS: mechanistic insights and therapeutic implications.” Autophagy 18(2):254-282.
- Deneubourg et al. (2022) “The spectrum of neurodevelopmental, neuromuscular and neurodegenerative disorders due to defective autophagy.” Autophagy 18(3): 496-517.
- Patergnani et al. (2021) “Understanding the Role of Autophagy in Cancer Formation and Progression Is a Real Opportunity to Treat and Cure Human Cancers.” Cancers (Basel) 13(22):5622 (Epub).
- Klionsky et al. (2021) “Autophagy in major human diseases.” EMBO J 40(19):e108863 (Epub).
- Kumar et al. (2021) “Autophagy and the Lysosomal System in Cancer.” Cells 10(10):2752 (Epub).
- Kocak et al. (2022) “Targeting autophagy in disease: established and new strategies.” Autophagy 18(3):473-495.
- Pandey et al. (2021) “Unfolding the role of autophagy in the cancer metabolism.” Biochem Biophys Rep 28:101158 (Epub).
- Liu and Du (2021) “Influence of Autophagy Inhibition on Lung Adenocarcinoma Cell Migration and Invasion Ability, and Efficacy of Gefitinib.” Technol Cancer Res Treat 20 (Epub).
- Yamamoto et al. (2021) “NEK9 regulates primary cilia formation by acting as a selective autophagy adaptor for MYH9/myosin IIA.” Nat Commun 12:3292 (Epub).