Detailed Guideline on Pyroptosis Research
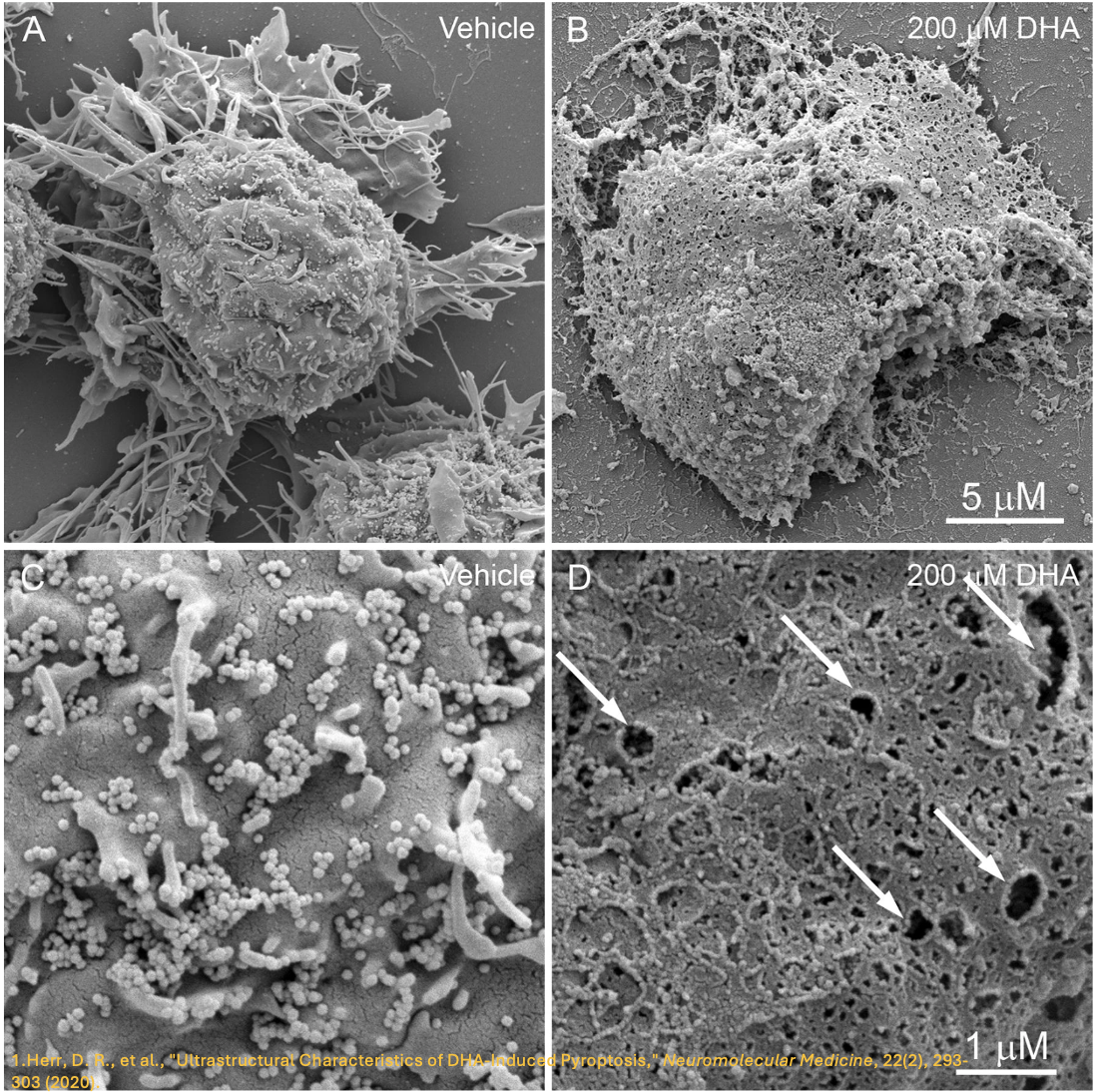
It is evident that the gasdermin family is central to pyroptosis. This is because the gasdermin family members are the executioners of pyroptosis, and their pore-forming function is a necessary condition for pyroptosis to occur. This family generally includes a cytotoxic N-terminal domain and a C-terminal inhibitory domain. After cleavage, the N-terminal domain is released and can assemble into pores in the membrane. Gasdermin pores disrupt the integrity of the cell membrane, leading to inflammatory cell death, with cell contents, including inflammatory cytokines, being released into the extracellular space.
In the 1980s and 1990s, scientists discovered that macrophages exposed to bacterial toxins or infections underwent a unique form of cell death that required the activation of caspase-1. For years, this process was mistakenly classified as apoptosis, a non-inflammatory and orderly form of cell death. However, unlike apoptosis, this newly observed cell death was marked by swelling, rupture, and the release of inflammatory signals, indicating a far more chaotic and immune-activating process. It wasn’t until 2001 that Cookson BT and Brennan MA officially named this inflammatory form of cell death “pyroptosis”. [2] Since then, research has revealed that pyroptosis plays a key role in immune defense by promoting inflammation in response to infections. The discovery of gasdermin D (GSDMD), a protein that forms pores in the cell membrane, further clarified the mechanism of pyroptosis. Today, it’s understood that pyroptosis occurs in various cell types and is critical in both fighting infections and contributing to inflammatory diseases when dysregulated.
Targeting Epigenetic Marks: How Antibodies Empower Cancer Research
Introduction
In the realm of genetics, the discovery of DNA and its double helix structure led to the assumption that genetic sequences alone determine cell phenotypes. However, researchers began to observe cases where organisms with identical genetic information exhibited different traits. This realization gave birth to the field of epigenetics, which explores the reversible influence on gene expression without altering DNA sequences. Epigenetic mechanisms encompass DNA methylation, histone modification, chromatin remodeling, and the effects of noncoding RNA. These processes involve “writers,” “readers,” and “erasers” that add, recognize, or remove chemical groups from DNA or histones. The cooperation between the epigenome, transcription factors, noncoding RNAs, and external stimuli regulates gene expression in a temporary yet long-lasting manner. Understanding normal and abnormal epigenetic processes is crucial for comprehending diseases like cancer and developing potential treatments.