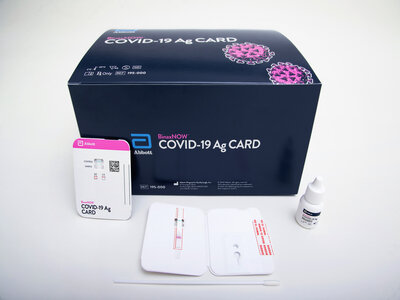
In a press release on August 26th, 2020, Abbott Laboratories announced that they were issued an emergency use authorization (EUA) by the FDA for their new, rapid point-of-care COVID-19 antigen test. Branded as the “BinaxNOW COVID-19 Ag Card”, the test is unique compared to the more prevalent molecular-based detection tests for COVID-19 in that it utilizes a lateral flow assay, similar to traditional over-the-counter pregnancy tests. Contained in a portable, credit-card sized device, Abbott claims that the test can deliver results in as little as 15 minutes, representing a significant reduction in time compared to RT-PCR-based tests that have turnaround times on a scale of hours rather than minutes.