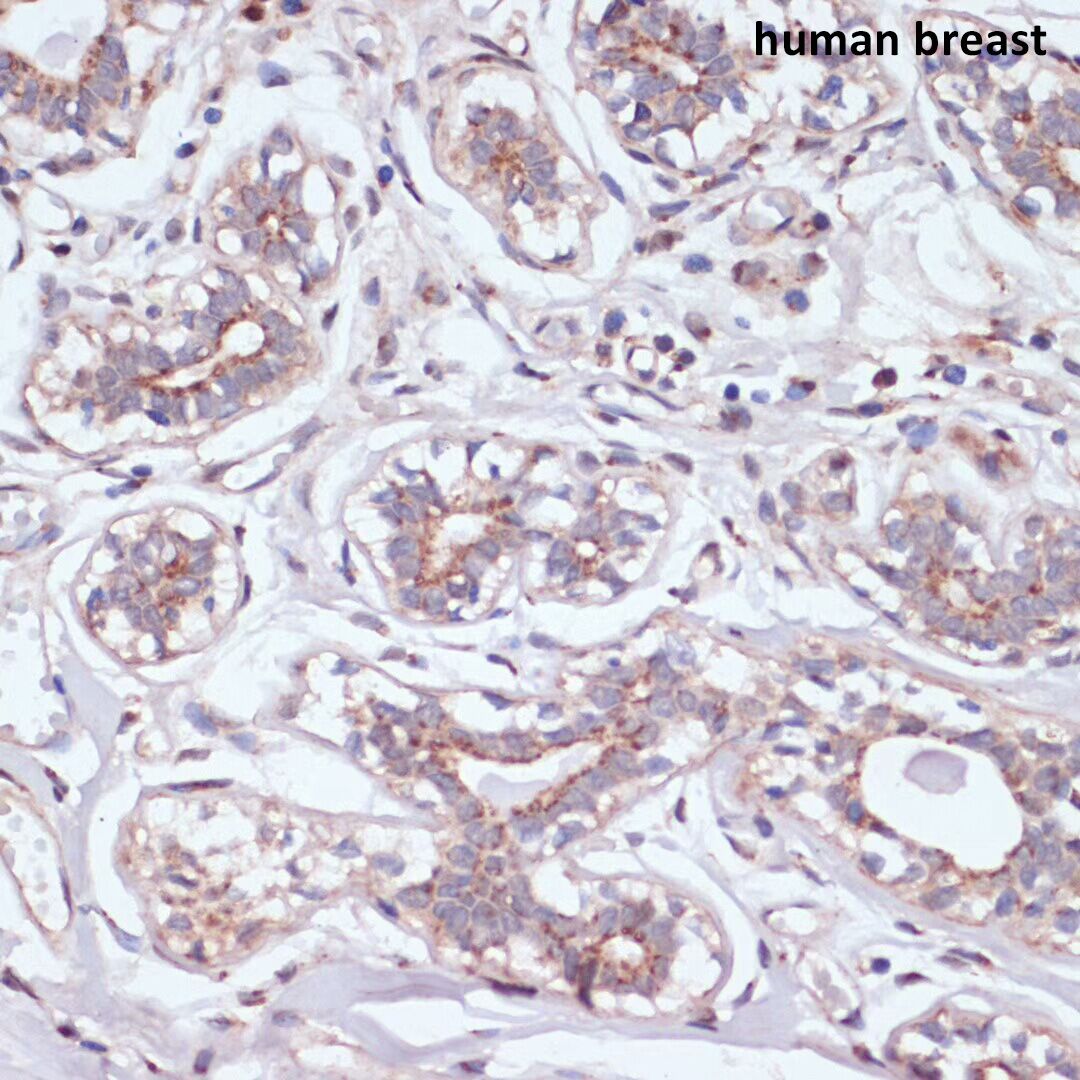
Epidermal growth factor receptor (EGFR, also known as ErbB-1 or HER1) is a member of the ErbB family. This family includes four tyrosine receptor kinases: HER1 (ErbB1, EGFR), HER2 (ErbB2, NEU), HER3 (ErbB3), and HER4 (ErbB4). The ErbB family plays an important regulatory role in the process of cell physiology, and is among the most studied receptor tyrosine kinases and signaling molecules in the history of biochemistry and cell biology.
EGFR is distributed along the surface of cells including mammalian epithelial cells, fibroblasts, glial cells, keratinocytes, and more. The EGFR signaling pathway plays an important role in physiological processes such as cell growth, proliferation and differentiation. Upon ligand binding (for example, EGF interacting with the extracellular domain of EGFR), the ErbB receptor tyrosine kinases will homodimerize or heterodimerize, allowing autophosphorylation of cytosolic tyrosine residues and the recruitment of downstream signaling molecules.
The loss of function in tyrosine kinases such as EGFR, or the abnormal activity/cell localization of key factors in related signaling pathways, such as the p38 MAPK pathway, can cause multiple cancer types, diabetes, immunodeficiency and cardiovascular diseases. In modern medicine, typical treatment strategies include targeting the tyrosine kinase activity of the ErbB receptor with small molecular inhibitors, or humanized antibodies that will target cells that have overexpressed the ErbB receptor, such as using an anti-HER2 therapy to treat breast cancer.