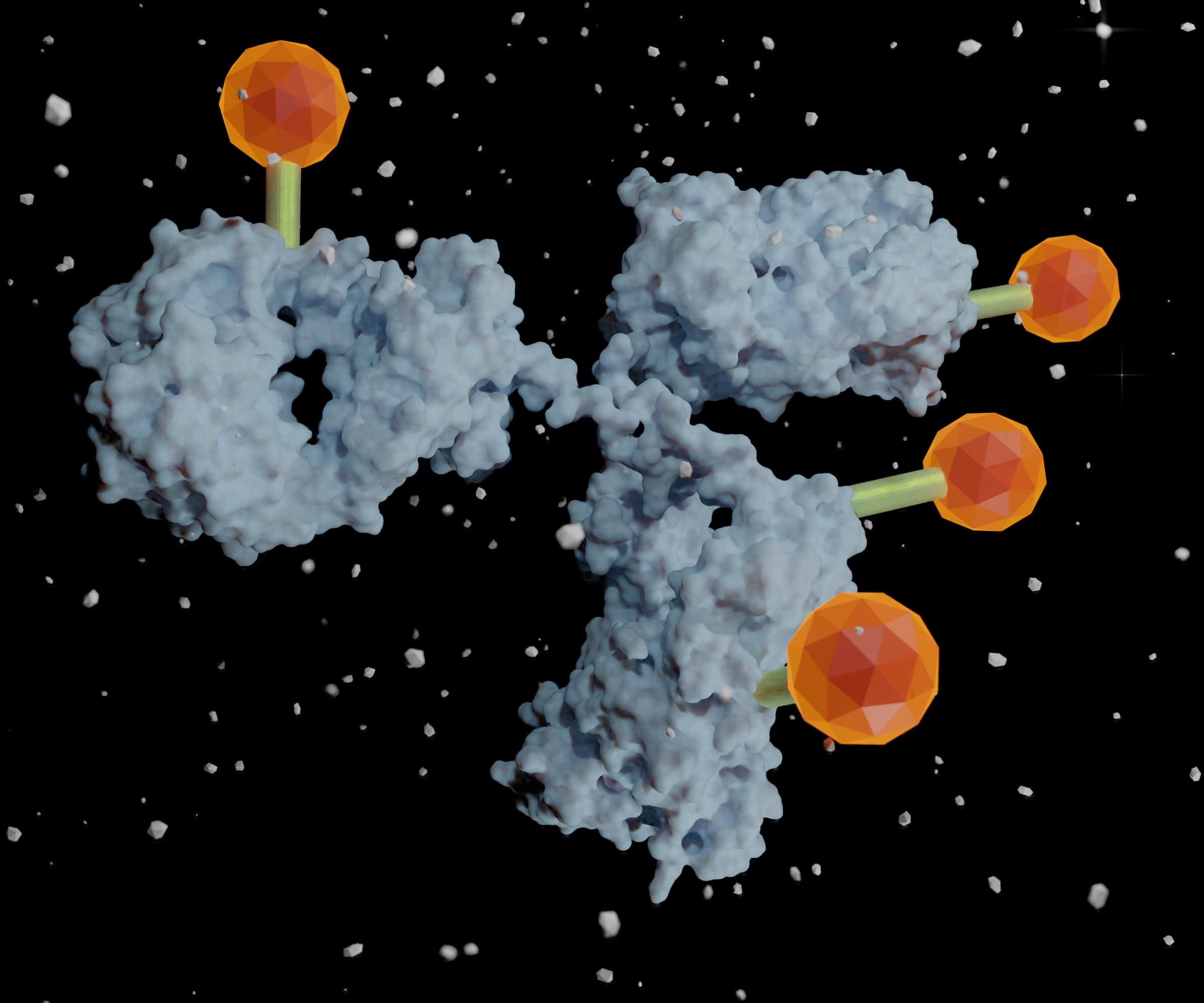
The dream of biomedical researchers is to fine-tune their therapeutics to precisely target the specific illness or pathogen affecting their patient. Ever since Nobel laureate and oft-quoted father of immunology, Paul Ehrlich, coined the term “magic bullet,” medical science has marched towards more personalized drugs that target key molecules that cause diseases including cancer. 1 We find ourselves now, over a century later, in an exciting era of discovery that has produced many antibody drug conjugates (ADC) designed to precisely target the diseased cells and not healthy cells. ADC uses this strategy to take advantage of the specificity of antibodies while delivering a covalently linked cytotoxic payload directly to diseased tissues to reduce the multitudes of side effects and toxicity. 2, 3 As basic research identifies more targets and antibody engineering procedures improve, the range of antitumor and anti-disease weapons may seem limitless.
The Magic Bullet: Current State of the Antibody-Drug Conjugate Market
As science advances, one of the recent trends that continues to pay dividends is immunotherapy to fight cancers. In many cases, the strategy is to mobilize the immune system to attack tumor cells based on cancer-specific antigens expressed either by the tumor itself or within the tumor microenvironment, either by stimulating normal immune cell function to their new tumor target or by removing the suppression of the immune system that is characteristic of many cancers. The trick is to find a way to attack only the tumor and not normal tissues, and certainly not to somehow trigger autoimmunity.
In the holiday rush, there were some fun science stories I was unable to get to other than the 2022 breakthrough of the year celebrating the ongoing JWST expedition. Now that we're back from celebrating with friends and family, let's check out some of what we missed!
What is STAT5B?
STAT5B belongs to the STAT (signal transducer and activator of transcription) protein family, a group of latent cytosolic transcription factors activated by Janus kinase (JAK) tyrosine kinases. The JAK-STAT signaling pathway is responsible for many important biological processes including cell proliferation, differentiation, apoptosis, and is also involved in the modulation of a variety of cytokines to control the immune response.
With a background in both immunology and cancer biology, I’ve always had a fascination with the interplay between the body’s immune system and any tumors that might pop up. Originally, it made sense that the immune system would actively seek out and destroy cancerous cells, but the emerging consensus is that the interactions between cancers and host immunity is far more complex. In addition to growing new blood vessels and reprogramming metabolic processes, there appears to be some imbalance between avoiding immune cells while also promoting tumor-infiltrating inflammatory cells to promote its growth. 1 (Figure 1) Trying to dissect this apparent contradictory relationship between tumors and host immunity remains a hot topic.
More Than a Feeling: The Science and Applications of Sensory Receptors
The 2021 Nobel Prize in Physiology of Medicine was awarded jointly to David Julius, of the University of California at San Francisco, and Ardem Patapoutian, a neuroscience researcher at the Scripps Research Institute in La Jolla, California. Working independently, Julius and Patapoutian discovered the key receptors (TRPV1, TRPM8, Piezo1, and Piezo2) in our bodies that sense heat, cold, and touch. Their work not only helps us to understand how we perceive and adapt to the surrounding world, but also paves the way for drug discoveries that target a wide range of diseases, including chronic pain, respiratory disease, and cancer.